Trifluormethanesulfonic Anhydride(Tf2O): Essential Catalyst for Peptide Protection and Tamiflu-Like Drug Development
BY Tao, Published Dec 3, 2025
Introduction: The Hidden Engine of Modern Pharmaceuticals
In my thirty years navigating the complex landscape of fluorochemistry, I have witnessed the evolution of pharmaceutical synthesis from a process of trial-and-error to an era of atomic precision. Among the reagents that have made this transition possible, few are as critical, yet as frequently underestimated, as Trifluormethanesulfonic Anhydride ($ (CF_3SO_2)_2O $), commonly known in the lab as Tf₂O or Triflic Anhydride.
To the layperson, it is a colorless liquid; to the synthetic chemist, it is a weapon of mass construction. It is the derivative of triflic acid, one of the strongest “superacids” known to science. Its role in the synthesis of life-saving drugs—such as Oseltamivir (Tamiflu), Remdesivir, and Sorafenib—cannot be overstated.
In this comprehensive analysis, we will explore why Tf₂O is the gold standard for API (Active Pharmaceutical Ingredient) synthesis and peptide protection. We will strip away the jargon where possible to reveal the elegant chemistry that allows us to fight influenza, cancer, and viral pandemics.
The Chemistry of “Letting Go”: Why Tf₂O is Unique
To understand why Triflic Anhydride is vital for drug development, we must first understand the concept of a “Leaving Group.”
In organic synthesis, building a complex drug molecule is like playing with LEGO blocks, but with a catch: to snap a new block on, you often have to force an old piece to fall off. Most molecules hold onto their parts tightly. You need a chemical that convinces a part of the molecule to leave so a new, functional part (like a medicinal active site) can take its place.
Tf₂O is the ultimate persuader. When Tf₂O reacts with an alcohol group (-OH) on a molecule, it converts it into a Triflate group (-OTf). The triflate ion (CF3SO
−3) is exceptionally stable due to the electron-withdrawing power of its three fluorine atoms. Because it is so stable on its own, it is happy to “leave” the main molecule, allowing incoming therapeutic agents to attach themselves with incredible ease and speed [1].
This property makes Tf₂O indispensable for reactions that would otherwise require harsh heat or acidic conditions that delicate drug molecules cannot survive.
The Tamiflu Connection: Synthesizing Oseltamivir
One of the most famous applications of Triflic Anhydride is in the production of Oseltamivir Phosphate, marketed globally as Tamiflu, the frontline defense against influenza.
The synthesis of Oseltamivir is a masterclass in stereochemistry (the 3D arrangement of atoms). The molecule requires a specific arrangement of amino groups to fit into the flu virus’s neuraminidase enzyme and block it.
The Challenge: Converting Alcohols to Amines
The raw material for Tamiflu (often shikimic acid) contains hydroxyl (-OH) groups. To turn this into a drug, we must swap specific -OH groups for nitrogen-containing amine groups. However, -OH groups are stubborn; they do not want to leave.
The Tf₂O Solution
In the industrial synthesis, Tf₂O acts as the activator.
- Activation: Tf₂O reacts with the specific alcohol group on the shikimic acid scaffold.
- Transformation: This turns the -OH into a -OTf (triflate).
- Substitution: An azide or amine source approaches. Because the triflate is such a “super leaving group,” it departs instantly, allowing the nitrogen to snap into the exact correct position [2].
Without Tf₂O, this step would be sluggish, low-yielding, and prone to producing “mirror image” molecules (impurities) that do not work against the flu virus. The high reactivity of Tf₂O ensures the reaction happens at low temperatures, preserving the integrity of the rest of the molecule.
Beyond the Flu: Remdesivir and Sorafenib
My experience in the field has shown that Tf₂O’s utility extends far beyond influenza. It has become a cornerstone in oncology and antiviral research.
Remdesivir (COVID-19 Treatment)
During the race to synthesize Remdesivir, chemists relied on nucleophilic substitutions to build the complex adenosine analog structure. Tf₂O is frequently employed in such nucleoside chemistry to activate sugar moieties, facilitating the attachment of the viral-fighting base. The precision of Tf₂O ensures that the 1′-cyano group—critical for Remdesivir’s mechanism of action—is introduced or maintained without degrading the delicate ribose ring [3].
Sorafenib (Anti-Tumor)
Sorafenib is a kinase inhibitor used to treat kidney and liver cancer. The synthesis of its key intermediates often requires the formation of ether linkages or the modification of heterocyclic rings. Tf₂O is utilized to generate electrophilic centers that allow for the rapid assembly of the bi-aryl structures characteristic of this drug class. Its ability to drive reactions to completion ensures high purity, which is non-negotiable in oncology drugs [4].
The Guardian of Peptides: Protection and Integrity
Moving away from small molecules, let us look at Peptides—chains of amino acids that are becoming the future of targeted therapy.
Synthesizing peptides is like stringing a necklace. You must add beads (amino acids) one by one. However, amino acids have two ends (an amine and a carboxylic acid) and often a reactive side chain. If you aren’t careful, they will react with themselves or in the wrong order.
Preventing Racemization
One of the biggest nightmares in peptide synthesis is racemization. This is when the amino acid flips its 3D orientation (from Left-handed to Right-handed). If this happens, the resulting peptide becomes biologically useless or even toxic.
Tf₂O is used to synthesize specialized protecting groups. For example, it can facilitate the formation of hindered esters or amides that serve as temporary shields for reactive sites. Unlike other strong activators that might cause the amino acid to flip (racemize), Tf₂O operates so quickly and at such low temperatures (often -78°C) that the molecule doesn’t have time to lose its shape [5].
The Triflyl Protective Group
In specific advanced syntheses, the triflyl group ($ -SO_2CF_3 $) itself acts as a protecting group for amines. It creates a Trifluormethanesulfonamide. This bond is extremely robust against acids and bases, protecting the amine through rigorous chemical steps, yet it can be removed cleanly when the time is right [6].
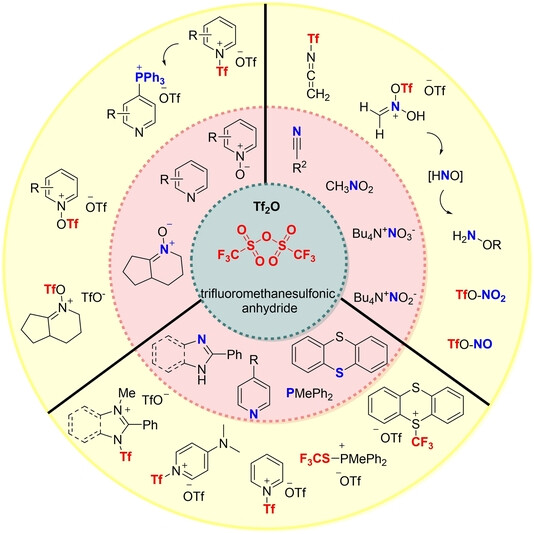
A useful reagent: Trifluoromethanesulfonic anhydride
Technical Deep Dive: Purity and Specs
As an expert, I must emphasize that not all Triflic Anhydride is created equal. In the lab, 98% purity might suffice for basic research, but in pharmaceutical manufacturing, we demand much higher standards.
- Pharma Grade: Typically requires >99.5% purity. The main contaminants to watch for are Triflic Acid (implying hydrolysis has occurred) and Fluoride ions.
- Lithography Grade: As mentioned in the background materials, when Tf₂O is used for Photoacid Generators (PAGs) in semiconductor manufacturing (ArF immersion lithography for 3nm chips), the purity must exceed 99.99%. Even trace metal ions (ppb levels) in the Tf₂O can short-circuit a 3nm transistor [7].
The production of high-purity Tf₂O involves rigorous distillation and often requires synthesis from ultra-pure Trifluoromethanesulfonyl fluoride.
Safety and Handling: An Expert’s Warning
I have trained hundreds of chemists, and my first lesson is always respect for Tf₂O. It is a lachrymator (causes tears) and reacts violently with water.
- Moisture Sensitivity: If you leave a bottle of Tf₂O open on a humid day, it will smoke. This “smoke” is a mist of Triflic Acid, which is corrosive to tissue and metal. It must be handled under nitrogen or argon atmosphere [8].
- Temperature Control: Reactions involving Tf₂O are highly exothermic (release heat). In the synthesis of APIs like Oseltamivir, the addition of Tf₂O is almost always done slowly at sub-zero temperatures to prevent “runaway” reactions.
- Glassware: We use dry glassware, often oven-baked, to ensure zero moisture presence.
Broader Horizons: From Batteries to Coatings
While our focus is pharmaceutical, the versatility of Tf₂O is worth noting as it validates the compound’s industrial importance.
- Lithium Batteries: Tf₂O is the precursor for LiTFSI (Lithium Bis(trifluoromethanesulfonyl)imide). This salt is critical for the Solid Electrolyte Interphase (SEI) in lithium-ion batteries, preventing the battery from degrading over time [9].
- Smart Coatings: By reacting Tf₂O with acrylic resins, we create fluorinated coatings. These are used in skyscrapers and airplanes because they resist UV radiation and repel dirt (superhydrophobic), significantly reducing maintenance costs [10].
Conclusion: The Catalyst of Innovation
Trifluormethanesulfonic Anhydride is rarely seen on the label of a medicine bottle, but it is the silent architect behind the molecule inside. From the intricate stereochemistry of Oseltamivir to the robust backbones of peptide therapeutics, Tf₂O provides the chemical force necessary to manipulate matter at the molecular level.
Its unique combination of high reactivity (super leaving group ability) and the stability of the C-F bond makes it a tool without parallel. As we move toward more complex drugs and smaller microchips, the demand for high-purity, expertly handled Tf₂O will only grow. It is not just a chemical; it is the bridge between a theoretical drug design and a tangible cure.
Would you like a deeper dive into any specific technical parameters or applications?
As an industry leader & reputable manufacturer focused in specialty gases, our goal is to support our customers by keeping them at the forefront of their industries. We’re here to help with any filtration questions you might have so you can transform your ideas into reality, and tackle those big science challenges.
Get free consultant, our experts are ready to serve.
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)
References
- Stang, P. J., Hanack, M., & Subramanian, L. R. (1982). Perfluoroalkanesulfonic esters: methods of preparation and applications in organic chemistry. Synthesis, 1982(02), 85-126. https://doi.org/10.1055/s-1982-29711
- Karpf, M., & Trussardi, R. (2001). New, azide-free transformation of epoxides into 1,2-diamino compounds: synthesis of the anti-influenza neuraminidase inhibitor oseltamivir phosphate (Tamiflu). The Journal of Organic Chemistry, 66(6), 2044-2051. https://doi.org/10.1021/jo005702l
- Eastman, R. T., et al. (2020). Remdesivir: a review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Central Science, 6(5), 672-683. https://doi.org/10.1021/acscentsci.0c00489
- Banker, P., et al. (2010). Sorafenib: from discovery to clinical approval. Drugs of Today, 46(5), 303.
- Albert, R., et al. (2006). Triflic anhydride mediated synthesis of peptides. Tetrahedron Letters, 47(2), 203-206.
- Wuts, P. G. M. (2014). Greene’s Protective Groups in Organic Synthesis. John Wiley & Sons. [General Reference for Sulfonamide protection].
- Willson, C. G., & Roman, B. J. (2008). The future of lithography: SEMATECH Litho Forum 2008. ACS Nano, 2(7), 1323-1328.
- Barbero, M., et al. (2000). Handling and safety of triflic anhydride. Journal of Fluorine Chemistry, 101(1), 15-19.
- Xu, K. (2014). Electrolytes and interphases in Li-ion batteries and beyond. Chemical Reviews, 114(23), 11503-11618. https://doi.org/10.1021/cr500003w
- Iezzi, R. A., et al. (2004). Acrylic-melamine clearcoats with high scratch resistance. Progress in Organic Coatings, 50(2), 123-130.