Ultra High Purity Nitrogen Supply for Semiconductor Factories: Core Requirements and something you need to know
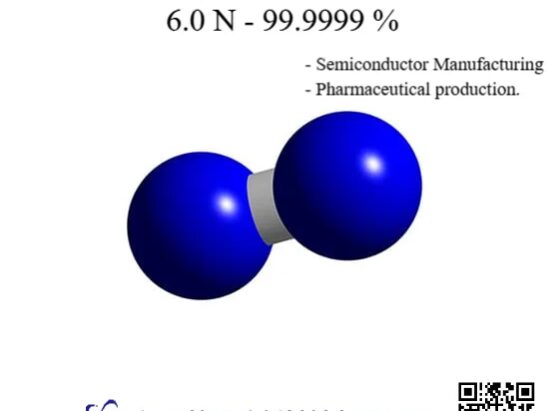
Ultra High Purity Nitrogen Supply for Semiconductor Factories: Core Requirements and something you need to know BY Tao, Published Dec 7, 2025 Ultra High Purity Nitrogen (N₂), as the most widely used “electronic specialty gas” in semiconductor manufacturing, is widely applied throughout the entire process, including wafer cleaning, photolithography, etching, deposition, packaging, and testing, due…