Methane in Deep Space Exploration: Closed-Loop CH4/O2 Propulsion and In-Situ Fuel Production for Lunar and Martian Bases
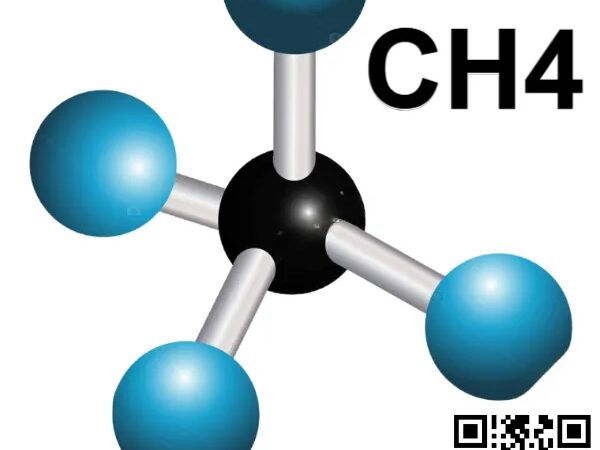
Methane in Deep Space Exploration: Closed-Loop CH4/O2 Propulsion and In-Situ Fuel Production for Lunar and Martian Bases BY Tao, Published Jan 18, 2026 Methane (CH4), a simple hydrocarbon gas, is emerging as a game-changer in deep space propulsion and sustainment. Its pairing with oxygen (O2) in closed-loop systems offers unprecedented efficiency, while in-situ production—making…