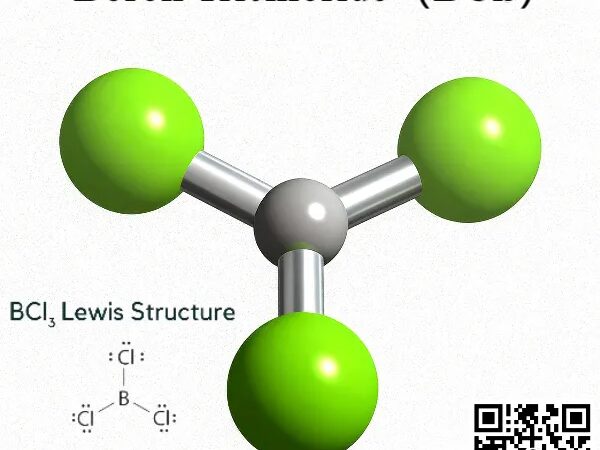
What Makes BCl₃ the Go-To Plasma Etching Gas for Nano-Chip Manufacturing?
What Makes BCl₃ the Go-To Plasma Etching Gas for Nano-Chip Manufacturing? BY Tao, Published Oct 11, 2025 Introduction: The Invisible Force Powering Your Digital World As an industry veteran with over 10+ years of hands-on research in specialty gases—from rare gases like xenon to reactive fluorocarbons—I’ve witnessed firsthand how seemingly obscure compounds drive technological…