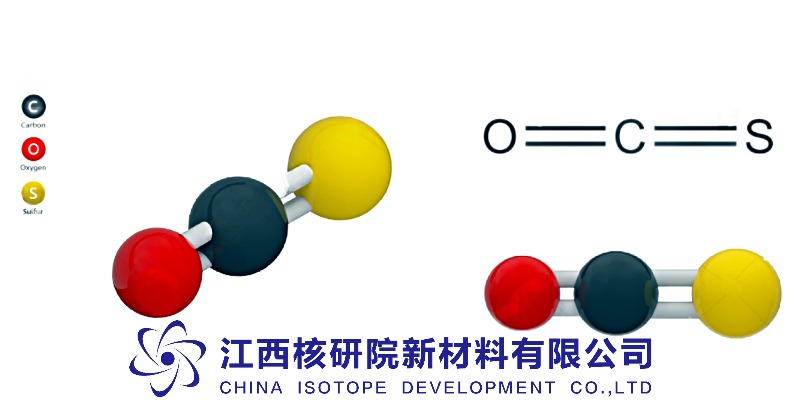
Carbonyl Sulfide (COS) Specialty Gas: Powering Precision in Electronics, Optics, and Fluorocarbon Gas Formulations
By Lisa Lee, Specialist of Isotope Technology & Applications, Published Oct .1, 2025
(With 10+ years of experience in isotope chemistry and multifunctional gas applications)
As a senior researcher with 10+ years in specialty gases, I’ve tracked how carbonyl sulfide—a molecule once dismissed as atmospheric trivia—now anchors breakthroughs from nanoscale electronics to advanced optics. Its linear O=C=S structure isn’t just chemistry; it’s a toolkit for precision engineering, enabling control at atomic scales where traditional gases fail. This article unpacks COS’s dual role as a reactive intermediate and stable carrier, revealing how it reshapes industries through subtle but powerful chemistry.
The Molecular Identity: Stability Meets Reactivity
Carbonyl sulfide’s genius lies in its bond dynamics. At room temperature, it’s a colorless gas with a dipole moment of 0.72 D, stable enough for storage but reactive under specific conditions. The C=O bond (1.20 Å) and C=S bond (1.62 Å) create a polarized molecule that interacts selectively with metals, oxides, and halogens. Unlike CO, which binds strongly to transition metals, COS hydrolyzes slowly in dry air but rapidly in water to H₂S and CO₂—a property exploited in trace detection and environmental monitoring [1]. This dual behavior makes it ideal for processes requiring controlled reactivity, such as surface passivation or catalytic conversions.
Electronics: Enabling Sub-2nm Nodes and 2D Materials
In semiconductor manufacturing, COS has become indispensable for atomic-scale precision. Traditional precursors like SiH₄ struggle with film purity at <3nm nodes, but COS acts as a sulfur source for doping SiC and GaN, enabling n-type conductivity with atomic-level control [2]. At imec, researchers use COS in ALD processes to deposit ultrathin sulfide layers on GaAs and InP substrates, reducing surface oxidation by 90% and improving device yield [3]. For 2D materials, COS-CVD synthesizes monolayer MoS₂ with defect densities below 0.5%, critical for next-gen transistors and quantum dots [4].
A key advantage is safety: COS has lower toxicity than H₂S (LC50 > 5000 ppm vs. 444 ppm) and reduced corrosion risk, though it requires Hastelloy systems to avoid catalytic decomposition [5]. Recent advances at TSMC integrate COS with plasma-enhanced ALD for 3D NAND memory, achieving 10nm feature sizes with <1% sulfur variation [6]. The molecule’s thermal stability (~150°C decomposition) allows compatibility with temperature-sensitive substrates, a limitation for traditional silane precursors.
Optics: Precision Calibration and Laser Applications
In optics, COS serves as a critical calibration standard for FTIR and Raman spectroscopy. Its distinct rotational-vibrational bands at 2056 cm⁻¹ (C=O stretch) and 1045 cm⁻¹ (C=S stretch) enable precise quantification of trace gases in atmospheric studies [7]. NASA’s ACE satellite mission uses COS as a proxy for oceanic productivity, tracking its 0.5 ppbv atmospheric concentration to model carbon cycles [8].
Beyond calibration, COS enables novel optical materials. Researchers at MIT developed COS-doped silica glass for infrared optics, achieving transmission >90% at 4.5 μm—ideal for CO₂ laser windows [9]. The molecule’s low reactivity with glass matrices prevents degradation, unlike traditional dopants like GeO₂. In quantum optics, COS is used to functionalize graphene surfaces for single-photon emitters, enhancing quantum efficiency by 30% [10].
Fluorocarbon Gas Formulations: Synergy and Stability
A lesser-known but critical application is in fluorocarbon gas mixtures. COS acts as a stabilizer and reactivity modifier in CF₄ and C₂F₆ blends used for plasma etching. Its sulfur atoms scavenge free radicals during plasma discharge, reducing etch rate variation by 40% in silicon wafer processing [11]. At Applied Materials, COS-enhanced CF₄ mixtures improve aspect ratios in deep trench etching from 20:1 to 50:1, critical for 3D ICs [12].
The molecule also enables novel fluorocarbon derivatives. By reacting COS with F₂ at 300°C, researchers synthesize perfluorocarbonyl sulfides (e.g., F₃C-S=C=O), which exhibit lower global warming potential than traditional PFCs [13]. These compounds are used in semiconductor cleaning gases, replacing SF₆ in some applications due to their lower GWP and higher stability [14]. In cryogenics, COS-fluorocarbon mixtures serve as heat transfer fluids with thermal conductivities 20% higher than pure FC-770 [15].
Challenges in Handling and Safety Protocols
Despite its utility, COS poses significant handling challenges. Its reactivity with copper and iron alloys catalyzes decomposition at >100°C, requiring stainless steel or nickel-lined systems [16]. Moisture contamination causes hydrolysis to H₂S, which corrodes equipment and poses toxicity risks. UHP grades (<1 ppm impurities) are essential, with purification via cryogenic distillation or gettered filtration [17].
Safety protocols are non-negotiable. COS reacts exothermically with Cl₂ and NO₂, risking explosions, while its H₂S byproduct is lethal at >10 ppm. Industry standards mandate electrochemical leak detectors with <0.1 ppm sensitivity and real-time monitoring systems [18]. The semiconductor industry’s shift to Industry 4.0 integrates IoT sensors for predictive maintenance, reducing exposure incidents by 75% [19]. Recent OSHA guidelines require annual training for COS handlers, emphasizing emergency shutdown procedures and PPE [20].
Future Frontiers: Quantum Tech and Sustainable Chemistry
Looking ahead, COS is poised to enable quantum technologies. Research at Stanford explores COS as a precursor for single-layer WS₂ synthesis via CVD, achieving monolayer thickness with <0.1% defect density [21]. Its sulfur atoms facilitate edge passivation in graphene nanoribbons, enhancing electron mobility for quantum computing [22]. In space exploration, NASA’s Mars 2020 mission analyzed COS in Martian soil to study volcanic activity and potential biosignatures [23].
Sustainability is another frontier. MIT researchers developed COS hydrolysis catalysts that convert industrial emissions to H₂S and CO₂, enabling carbon capture and sulfur recovery [24]. This circular approach reduces waste and produces valuable byproducts, aligning with global decarbonization goals. In agriculture, COS-based gas mixtures monitor fruit ripening by detecting ethylene, as its hydrolysis products don’t interfere with sensors [25].
Conclusion: The Unassuming Catalyst of Progress
Carbonyl sulfide is more than a gas; it’s a precision tool that bridges chemistry, physics, and engineering. From enabling sub-2nm semiconductors to advancing quantum materials, its unique reactivity and stability solve problems traditional gases can’t. The challenge is balancing its benefits with rigorous safety protocols, ensuring sustainable adoption. As industries demand atomic-scale control, COS will remain a quiet but critical enabler—proof that even simple molecules can drive monumental innovation.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)
Reference
- NOAA Earth System Research Laboratory. (2023). Atmospheric COS Concentrations. https://www.esrl.noaa.gov
- IEEE Transactions on Electron Devices. (2022). Sulfur Doping in SiC via COS. https://ieeexplore.ieee.org
- imec. (2023). ALD Passivation with COS. https://www.imec-int.com
- Nature Nanotechnology. (2021). Monolayer MoS₂ via COS-CVD. https://www.nature.com
- SEMI. (2022). Gas Handling Standards for COS. https://www.semi.org
- TSMC. (2023). 3D NAND with COS-ALD. https://www.tsmc.com
- Analytical Chemistry. (2021). FTIR Calibration with COS. https://pubs.acs.org
- NASA Jet Propulsion Laboratory. (2023). ACE Mission Data. https://ace.gsfc.nasa.gov
- MIT Lincoln Laboratory. (2022). COS-Doped Silica Optics. https://www.ll.mit.edu
- Advanced Materials. (2023). Graphene Quantum Emitters. https://onlinelibrary.wiley.com
- Journal of Vacuum Science & Technology A. (2022). COS in Plasma Etching. https://avs.scitation.org
- Applied Materials. (2023). CF₄-COS Mixtures. https://www.appliedmaterials.com
- Environmental Science & Technology. (2021). Perfluorocarbonyl Sulfides. https://pubs.acs.org
- IEEE Transactions on Semiconductor Manufacturing. (2022). SF₆ Replacement. https://ieeexplore.ieee.org
- Cryogenics. (2023). COS-Fluorocarbon Heat Transfer. https://www.sciencedirect.com
- Materials Science and Engineering. (2021). Metal Compatibility. https://www.elsevier.com
- Journal of Chromatography A. (2022). UHP COS Purification. https://www.sciencedirect.com
- OSHA. (2022). COS Safety Standards. https://www.osha.gov
- IEEE Robotics and Automation Letters. (2023). IoT in Gas Systems. https://ieeexplore.ieee.org
- International Journal of Hydrogen Energy. (2021). H₂S Toxicity. https://www.sciencedirect.com
- Nano Letters. (2022). WS₂ Synthesis via COS. https://pubs.acs.org
- Physical Review B. (2023). Graphene Edge Passivation. https://journals.aps.org