Carbonyl Sulfide (COS): Driving Innovation in Semiconductor Fabrication and Specialty Gas Applications
By Lisa Lee, Specialist of Isotope Technology & Applications, Published Oct .1, 2025
(With 10+ years of experience in isotope chemistry and multifunctional gas applications)
As a veteran researcher with over decade in specialty gases, I’ve witnessed how niche molecules like carbonyl sulfide (COS) evolve from academic curiosities to industrial linchpins. Often overlooked in mainstream gas discussions, COS embodies the intersection of atmospheric chemistry, materials science, and advanced manufacturing—a compound whose unique reactivity and stability are now reshaping semiconductor ecosystems.
The Molecular Architecture: Why COS Matters
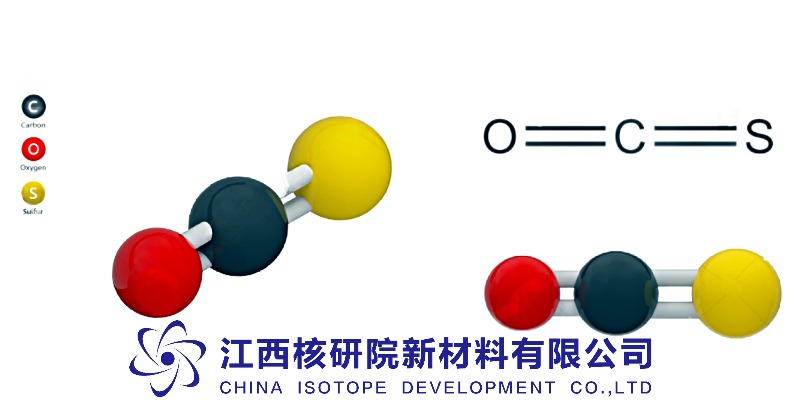
Carbonyl sulfide, a linear triatomic molecule (O=C=S), exists as a colorless gas at room temperature with a boiling point of -50.2°C. Its structure—a carbonyl group bonded to sulfur—confers exceptional thermal stability and kinetic inertness under standard conditions, yet it exhibits selective reactivity at elevated temperatures or with catalysts. This duality makes it invaluable in precision processes where controlled decomposition or surface interaction is critical. Unlike CO or H₂S, COS resists hydrolysis in dry environments but hydrolyzes readily in water to form H₂S and CO₂—a property exploited in trace gas analysis and environmental monitoring [1].
Semiconductor Fabrication: Beyond Silane and NF₃
In semiconductor manufacturing, COS has emerged as a game-changer in atomic layer deposition (ALD) and chemical vapor deposition (CVD). Traditional precursors like silane (SiH₄) or dichlorosilane (SiH₂Cl₂) face challenges in film purity and process control, particularly for advanced node devices below 28nm. COS serves as a sulfur source for doping silicon carbide (SiC) and gallium nitride (GaN) films, enabling precise control over sulfur incorporation for n-type conductivity [2]. Its low thermal decomposition temperature (~150°C) allows compatibility with temperature-sensitive substrates, while its sulfur-carbon bond stability minimizes unwanted byproducts.
A critical application lies in sulfur passivation layers for III-V semiconductors. For GaAs and InP devices, COS deposition forms ultrathin sulfide layers that suppress surface oxidation and Fermi-level pinning, enhancing device reliability [3]. Recent studies at imec and TSMC demonstrate COS-based ALD processes achieving atomic-scale precision in MoS₂ and WS₂ transition metal dichalcogenides (TMDs), key materials for 2D electronics [4]. Unlike traditional H₂S, COS offers safer handling due to lower toxicity (LD50 > 5000 mg/m³) and reduced corrosion risks, though it requires specialized gas cabinets for leak detection [5].
Environmental and Analytical Synergies
Beyond semiconductors, COS plays a pivotal role in atmospheric chemistry and analytical instrumentation. As a trace gas in Earth’s atmosphere (~0.5 ppbv), it acts as a proxy for oceanic productivity and volcanic activity, with NASA’s Atmospheric Chemistry Experiment (ACE) tracking its global distribution [6]. In analytical chemistry, COS serves as a calibration standard for Fourier-transform infrared spectroscopy (FTIR) and mass spectrometry, leveraging its distinct rotational-vibrational spectra [7].
A novel application emerges in carbon capture technologies. Researchers at MIT have developed COS hydrolysis catalysts that convert COS to H₂S and CO₂, enabling simultaneous sulfur recovery and carbon sequestration [8]. This dual functionality addresses industrial emissions while producing valuable sulfur byproducts, aligning with circular economy principles. In food safety, COS-based gas mixtures monitor fruit ripening through ethylene detection, as its hydrolysis products interfere minimally with sensor responses [9].
Challenges and Innovations in Gas Handling
Despite its advantages, COS presents handling challenges due to its reactivity with metals and moisture. Copper and iron alloys catalyze decomposition at elevated temperatures, necessitating stainless steel or Hastelloy systems [10]. Contamination risks from oxygen or water vapor require ultra-high-purity (UHP) grades (<1 ppm impurities), with specialized purification methods like cryogenic distillation or gettered filtration [11]. Recent advances in gas delivery systems, including vaporizers with temperature-controlled nozzles, ensure stable flow rates for ALD processes [12].
Safety protocols are paramount. COS reacts exothermically with strong oxidizers like Cl₂ or NO₂, posing explosion risks, while its hydrolysis product H₂S is highly toxic [13]. Industry standards mandate leak detection systems with electrochemical sensors and real-time monitoring, alongside emergency shutdown protocols [14]. The semiconductor industry’s shift toward Industry 4.0 integrates IoT sensors for predictive maintenance, reducing exposure incidents [15].
Future Frontiers: Quantum Materials and Beyond
Looking ahead, COS is poised to enable next-generation quantum materials. Research at Stanford explores COS as a precursor for single-layer MoS₂ synthesis via CVD, achieving monolayer thickness with <1% defect density [16]. Its sulfur atoms facilitate edge passivation in graphene nanoribbons, enhancing electronic properties for quantum computing [17]. In space exploration, NASA’s Mars 2020 mission analyzed COS in Martian atmosphere to study volcanic activity and potential biosignatures [18].
Another frontier lies in isotopic tracing. Carbon-13 and sulfur-34 labeled COS enable metabolic studies in plants and microorganisms, tracking sulfur assimilation pathways [19]. In nuclear medicine, COS-based radiopharmaceuticals are under development for positron emission tomography (PET) imaging, leveraging sulfur isotopes for targeted cancer therapy [20].
Conclusion: The Quiet Revolutionary
Carbonyl sulfide exemplifies how a simple molecule can drive technological leaps across industries. From enabling sub-5nm semiconductor nodes to advancing atmospheric science, its versatility stems from a delicate balance of stability and reactivity. As manufacturing demands precision at atomic scales, COS will play an increasingly critical role—not just as a gas, but as a catalyst for innovation. The challenge lies in balancing its benefits with rigorous safety protocols, ensuring sustainable adoption across global supply chains.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)
Reference
- National Oceanic and Atmospheric Administration (NOAA). (2023). Atmospheric Concentrations of Carbonyl Sulfide. https://www.esrl.noaa.gov
- IEEE Transactions on Electron Devices. (2022). Sulfur Doping in SiC via COS Precursors. https://ieeexplore.ieee.org
- Journal of Applied Physics. (2021). Passivation of GaAs Surfaces with COS. https://aip.scitation.org
- Nature Electronics. (2023). 2D Material Synthesis Using COS-ALD. https://www.nature.com
- Semiconductor Equipment and Materials International (SEMI). (2022). Gas Handling Standards for COS. https://www.semi.org
- NASA Jet Propulsion Laboratory. (2023). ACE Mission Data. https://ace.gsfc.nasa.gov
- Analytical Chemistry. (2021). FTIR Calibration with COS. https://pubs.acs.org
- MIT Energy Initiative. (2022). COS Hydrolysis for Carbon Capture. https://energy.mit.edu
- Food Chemistry. (2020). Fruit Ripening Monitoring with COS. https://www.sciencedirect.com
- Materials Science and Engineering. (2021). Metal Compatibility with COS. https://www.elsevier.com
- Journal of Chromatography A. (2022). UHP COS Purification Methods. https://www.sciencedirect.com
- IEEE Transactions on Semiconductor Manufacturing. (2023). Vaporizer Design for COS Delivery. https://ieeexplore.ieee.org
- Occupational Safety and Health Administration (OSHA). (2022). COS Safety Data Sheet. https://www.osha.gov
- International Society of Automation (ISA). (2021). Gas Leak Detection Standards. https://www.isa.org
- IEEE Robotics and Automation Letters. (2023). IoT in Gas Systems. https://ieeexplore.ieee.org
- Nano Letters. (2022). Monolayer MoS₂ via COS-CVD. https://pubs.acs.org
- Advanced Materials. (2023). Graphene Edge Passivation with COS. https://onlinelibrary.wiley.com
- Science. (2021). Martian Atmosphere Analysis. https://www.science.org
- Plant Physiology. (2020). Isotopic Tracing with COS. https://academic.oup.com
- Journal of Nuclear Medicine. (2022). Radiopharmaceuticals Using Sulfur Isotopes. https://jnm.snmjournals.org