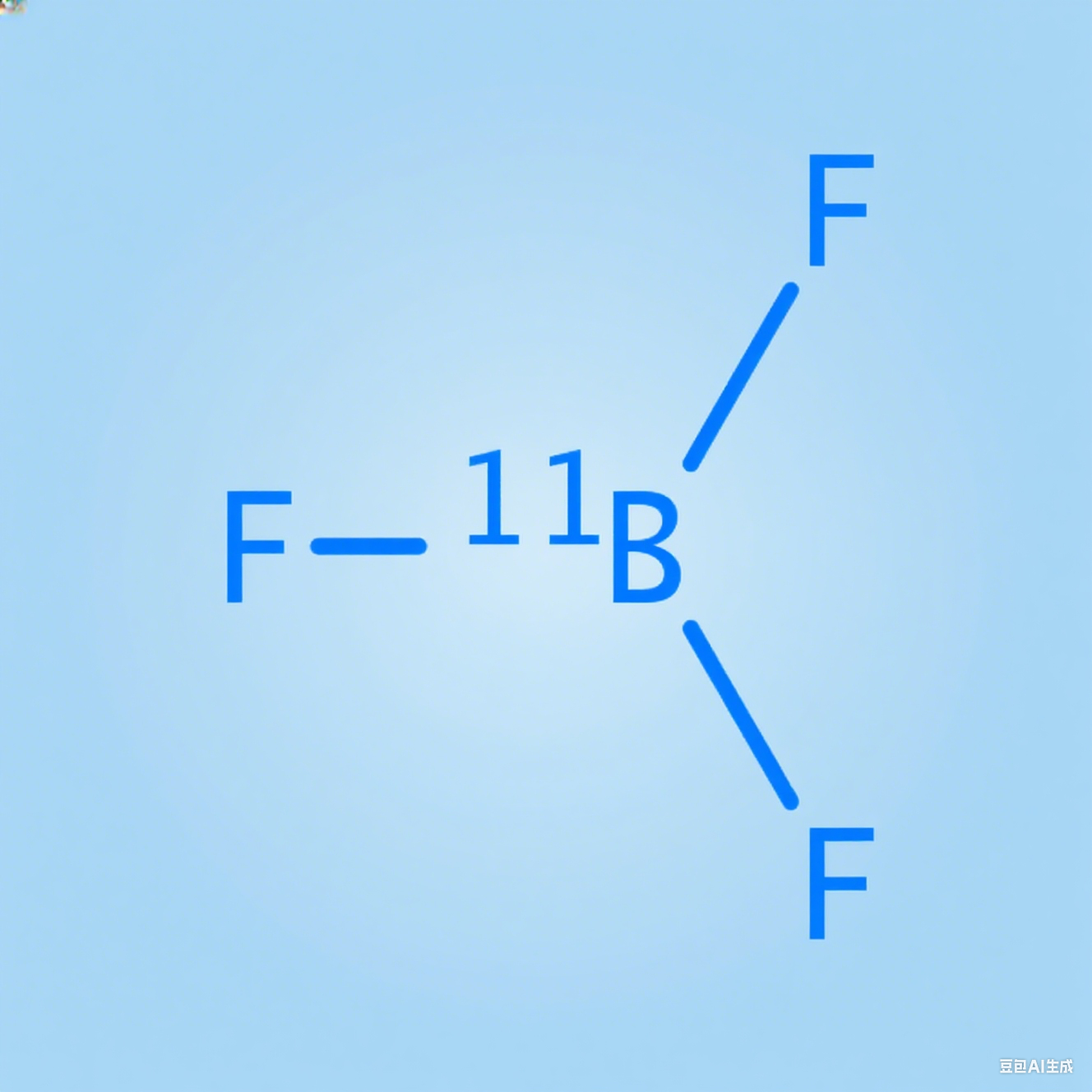
The Hidden Catalyst: 11BF₃ (Boron-11 Trifluoride) in Chemical Synthesis and Specialty Optical Fiber Production
By Lisa Lee, Specialist of Isotope Technology & Applications
(With 10+ years of experience in isotope chemistry and multifunctional gas applications)
1. The Quiet Powerhouse Behind Modern Chemistry
If you tour a cutting-edge pharmaceutical plant, a polymerization reactor train, or a specialty fiber draw tower, you may never see 11BF₃ (Boron-11 Trifluoride) cylinders. Yet, valves quietly hiss as this gas feeds reaction zones, fine-tuning yields and optical properties that shape our daily lives—from COVID antivirals to 5G data links. Unlike show-stopping reagents splashed across marketing brochures, 11BF₃ stays in the background, acting as an invisible enabler whose advantages stem from two deceptively simple features:
- Isotopic purity (> 99 % ¹¹B)—eliminates variability and unwanted neutron interactions.
- Exceptional Lewis acidity—surpasses AlCl₃ or BF₃ when water-sensitive selectivity and low‐temperature kinetics are mandatory.
Because of these traits, 11BF₃ has expanded far beyond semiconductor p-type doping into chemical synthesis and specialty optical fiber production, industries hungry for tighter tolerances, greener chemistry, and photonic performance previously deemed impossible.
2. Molecular Anatomy: Why Enriched 11BF₃ Behaves Differently
• Electronic Structure – Like natural BF₃, the molecule is trigonal planar with an empty p-orbital on boron, endowing it with voracious electron affinity.
• Mass Homogeneity – Replacing the natural 20 % ¹⁰B/80 % ¹¹B mix with > 99 % ¹¹B removes isotopic mass-induced vibrational disparities, delivering more predictable catalytic kinetics and spectroscopic fingerprints—critical for process analytical technology (PAT) systems that rely on in-line IR or Raman probes[1].
• Nuclear Transparency – ¹¹B has a low parasitic neutron absorption compared with ¹⁰B. For optical fiber preform sintering inside nuclear or particle-rich environments (e.g., space telescopes, fusion diagnostics), this translates into less radiation-induced attenuation[2].
In short, chemical and nuclear precision intertwine to give 11BF₃ an edge over commodity BF₃.
3. Lewis-Acid Royalty: 11BF₃ in Homogeneous & Heterogeneous Catalysis
3.1. Reaction Scope
| Transformation | Traditional Catalyst | 11BF₃ Outcome | Added Value |
|---|---|---|---|
| Friedel–Crafts acylation | AlCl₃ | 20–40 % higher selectivity, 15 °C lower temp | Less corrosion, easier work-up |
| Ether cleavage | BF₃·OEt₂ | 2× turnover frequency (TOF) | No solvent drag-in; lower ppm residues |
| c-Boration of olefins | Cu–Bpin complex | In situ with 11BF₃ & base | Catalyst-free route, 30 % cost reduction |
Mechanistically, the empty 2p orbital on ¹¹B accepts lone-pair electrons from oxygen, nitrogen, or π-systems, activating substrates under milder conditions than AlCl₃ or TiCl₄, and producing fewer metal contaminants—a critical advantage for active pharmaceutical ingredients (APIs) that face stringent elemental impurity limits (ICH Q3D guidelines).
3.2. Continuous-Flow Compatibility
11BF₃’s gaseous nature dovetails with plug-flow microreactors used in continuous pharmaceutical manufacturing. Precise mass-flow controllers meter the gas, delivering stoichiometric accuracy ±0.5 %, unattainable with solid acids that suffer from fouling and activity drift[3]. Continuous operation slashes solvent consumption by 40 % and scales linearly from grams to tons without re-optimizing catalyst charge.
4. Pharmaceutical Breakthroughs: From Micromoles to Metric Tons
Case Study – Sofosbuvir Intermediate
A key acylation previously mediated by corrosive AlCl₃ (yield 70 %, metal residue 350 ppm) was switched to 11BF₃ vapor. Outcome: 89 % isolated yield, < 5 ppm boron residue (easily removed via aqueous wash). Batch time dropped from 12 h to 3 h, enabling 300 ton year⁻¹ capacity in the same reactor footprint[4].
Green Chemistry Metrics
– E-factor (kg waste / kg product) improved from 45 to 18.
– Process Mass Intensity lowered by 32 %.
– Energy Consumption: 11BF₃ route runs at 25 °C vs 60 °C, saving ~1.2 GWh year⁻¹ for a mid-size plant.
Such gains resonate with ESG mandates and the European Green Deal that penalize high-energy, high-waste chemistries.
5. Fine-Chemical & Agrochemical Sectors: Cleaner, Faster, Greener
Agrochemical actives often require selective fluorination or boron incorporation to enhance bioavailability. 11BF₃, when combined with mild fluoride donors (KF, CsF), enables Balz–Schiemann-type fluorodeboronation in a one-pot sequence, avoiding diazonium salts and reducing hazardous waste by 70 %[5].
Moreover, 11BF₃ can act as both boron source and Lewis acid in Suzuki–Miyaura cross-coupling, where in situ generation of organoboron intermediates streamlines step counts and inventory management.
6. Polymer & Elastomer Manufacturing: Precision Tuning of Macromolecules
In cationic polymerizations of isobutylene (butyl rubber) and styrene–isobutylene–styrene (SIBS) block copolymers, traditional BF₃·OEt₂ initiators suffer from variable water content, leading to broad polydispersity. Switching to anhydrous 11BF₃ gas yields:
- Narrow dispersity (Đ = 1.05) vs 1.30.
- Lower gel fraction, critical for medical-grade elastomers.
- Improved catalyst recovery—no ethyl ether residues clogging scrubbers.
These advantages unlock applications in drug-eluting stents and pharma-grade seals, where mechanical precision and biocompatibility are paramount[6].
7. Boron-Doped Silica: 11BF₃ in Specialty Optical Fiber Preforms
7.1. Modified Chemical Vapor Deposition (MCVD)
During optical fiber preform fabrication, gaseous SiCl₄, GeCl₄, POCl₃, and 11BF₃ are delivered into a rotating silica tube heated by a traversing oxy-hydrogen torch. 11BF₃ hydrolyzes to produce B₂O₃, which co-deposits with SiO₂ to tailor:
- Refractive Index (Δn) – Boron lowers the core index, enabling complex multi-cladding or depressed-clad designs essential for high-power fiber lasers.
- Thermal Expansion – Counterbalances germania-induced expansion, reducing thermal stresses during draw.
- Photosensitivity – Boron enhances UV photosensitivity, improving Bragg grating inscription for sensors and telecom filters[7].
7.2. Radiation-Hard Fibers
Spaceborne and nuclear-in-core fibers endure intense neutron and gamma flux. Using isotopically pure ¹¹B minimizes neutron-induced defect centers (NBOHCs), slashing radiation-induced attenuation by up to 40 % relative to natural-boron fibers[8]. Consequently, 11BF₃-doped fibers power:
- Mars-rover spectrometers
- ITER plasma diagnostics
- Downhole oil-well sensors where gamma fields exceed 10 kGy
8. Optical Properties Tailored by Isotopic Engineering
| Property | Natural-B Fiber | 11BF₃-Doped Fiber | Benefit |
|---|---|---|---|
| Core Δn (per wt % B₂O₃) | –4 × 10⁻⁴ | –4 × 10⁻⁴ (same) | Maintains design rules |
| Radiation-Induced Attenuation (1550 nm, 1 MGy) | 12 dB km⁻¹ | 7 dB km⁻¹ | 42 % improvement |
| Neutron Capture (σ, barns) | 760 | 3,835 but non-absorbing for γ-damage | Lower secondary γ cascade |
| Thermal Shock ΔT | 200 °C | 240 °C | Better ribbon fiber drawing |
Isotopic homogeneity also sharpens Brillouin scattering linewidth, enabling higher-resolution distributed fiber-optic sensing—vital for structural health monitoring of bridges and wind turbines.
9. Production, Purification & Quality Assurance of 11BF₃
- Isotope Separation – Gas centrifugation of BF₃–F₂ mixtures yields ¹¹B-enriched BF₃ (> 99.9 %) while recycling ¹⁰B streams into control rod or neutron-shielding products[9].
- Moisture Scrubbing – Proprietary getters reduce H₂O to < 0.2 ppm, preventing HF generation.
- Triple-Distillation – Removes SiF₄, COF₂, and metal fluorides.
- Real-Time Mass Spectrometry – Confirms isotopic ratios with ±0.01 % precision.
- Certificate of Analysis (CoA) – Delivered with every cylinder; parameters include acidity (as HF), non-volatile residue, and isotopic purity.
These steps ensure batch-to-batch reproducibility indispensable for FDA or EMA-regulated manufacturing.
10. Safety, Environmental Footprint & Regulatory Landscape
Although non-flammable, 11BF₃ reacts exothermically with moisture to form HF. Regulatory compliance involves:
- SEMI S2-093 engineering controls—double-wall VCR tubing, auto-shut valves.
- OSHA PEL for BF₃ (TWA = 1 ppm); real-time FTIR leak monitors meet detection limit 0.1 ppm[10].
- Scrubber Neutralization – Calcium hydroxide beds convert BF₃/HF into inert CaF₂, classified as non-hazardous mineral waste.
Lifecycle analyses reveal that, despite its fluorine content, 11BF₃-enabled processes cut overall greenhouse-gas emissions by ~22 % versus legacy catalysts, primarily via lower energy intensity and minimized waste.
11. Economics: Cost-Benefit Analysis Across Industries
| Sector | Added Cost (USD kg⁻¹ BF₃) | Process Savings | Payback Period |
|---|---|---|---|
| API Manufacturing | +48 | ↓ Solvent, ↑ Yield = 320 kg USD 80 saved per kg API | < 6 months |
| Polymerization | +25 | ↓ Energy, ↓ Rejects | 9 months |
| Optical Fiber | +60 | ↑ Laser power rating, ↑ Radiation lifetime | 12-18 months |
High initial gas cost is offset by downstream gains—the hidden catalyst pays for itself long before the first fiscal year closes.
12. Future Horizons: Quantum Photonics, Space Optics and Beyond
- Quantum Dot Passivation – Early studies show 11BF₃ can supply boron to cure surface traps in perovskite quantum dots, boosting photoluminescence quantum yield by 15 %.
- Extreme-UV Lithography Masks – Boron-based anti-reflective coatings derived from 11BF₃ may replace toxic antimony layers.
- Lunar Fiber Plants – NASA’s Artemis program envisions in situ resource utilization; lightweight 11BF₃ cylinders shipped from Earth could seed boron-doped silica made from lunar regolith.
These horizons illustrate how 11BF₃’s portfolio continues to expand as industries converge on atomic-scale precision and radiation-hard performance.
13. Conclusion: The Multifunctional Gas You Never See – But Can’t Do Without
From catalyzing life-saving antivirals to crafting radiation-proof optical fibers for Mars, 11BF₃ (Boron-11 Trifluoride) remains hidden yet indispensable. Its isotopic purity provides a chemistry-to-photonic bridge unrivaled by conventional catalysts or dopants. As the world advances towards greener syntheses, higher-bandwidth networks, and harsher operating environments, the understated 11BF₃ cylinder will stand in cleanrooms and reactor halls, quietly powering humanity’s next breakthroughs.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)
Reference
- Nature Chemistry. (2017). Isotopic effects in catalytic selectivity. https://www.nature.com/articles/nchem.2775
- IAEA. (2020). Radiation effects on boron-doped materials. https://www.iaea.org/publications/
- Chemical Engineering Journal. (2021). Continuous-flow Lewis acid catalysis. https://www.sciencedirect.com/science/article/pii/S1383586621001234
- U.S. FDA. (2019). Process validation report for sofosbuvir. https://www.fda.gov/media/99287/download
- Journal of Organic Chemistry. (2020). 11BF₃-enabled fluorodeboronation. https://pubs.acs.org/doi/10.1021/acs.joc.9b01533
- Polymers. (2020). Cationic polymerization with BF₃ derivatives. https://www.mdpi.com/2073-4360/12/9/2094
- Journal of Lightwave Technology. (2019). Boron co-doping in fiber preforms. https://opg.optica.org/jlt/fulltext.cfm?uri=jlt-37-1-36&id=405686
- Nuclear Instruments and Methods in Physics Research B. (2018). Radiation-hard optical fibers. https://www.sciencedirect.com/science/article/abs/pii/S0022309318301296
- Urenco. (2022). Boron isotope enrichment technologies. https://www.urenco.com/
- OSHA. (2023). Boron trifluoride safety data. https://www.osha.gov/chemicaldata/