C4F7N Gas: A Low-GWP Alternative to SF6 for Advanced Electrical Insulation and Arc Quenching
BY Tao, Published August 27, 2025
Introduction to C4F7N Heptafluoroisobutyronitrile
Perfluoroisobutyronitrile (C4F7N), a fluorinated specialty gas, is emerging as a game-changer in high-voltage electrical insulation and arc quenching, offering a sustainable alternative to sulfur hexafluoride (SF6). With over three decades of experience in specialty gases, I have observed the transformative potential of compounds like C4F7N in addressing environmental and technical challenges in modern industries. SF6, long the gold standard for electrical insulation due to its excellent dielectric properties, has a significant drawback: its global warming potential (GWP) is 23,500 times that of CO2, making it a target for replacement in eco-conscious applications. C4F7N, with a GWP of approximately 2,100, provides a lower environmental impact while maintaining comparable performance, positioning it as a cornerstone for sustainable electrical systems.
This article explores the properties, production, applications, and market significance of C4F7N, emphasizing its role in high-voltage equipment, semiconductor manufacturing, and other cutting-edge fields. By combining technical depth with accessible explanations, we aim to highlight why C4F7N is poised to redefine the standards of electrical insulation and arc quenching.
Chemical and Physical Properties of C4F7N
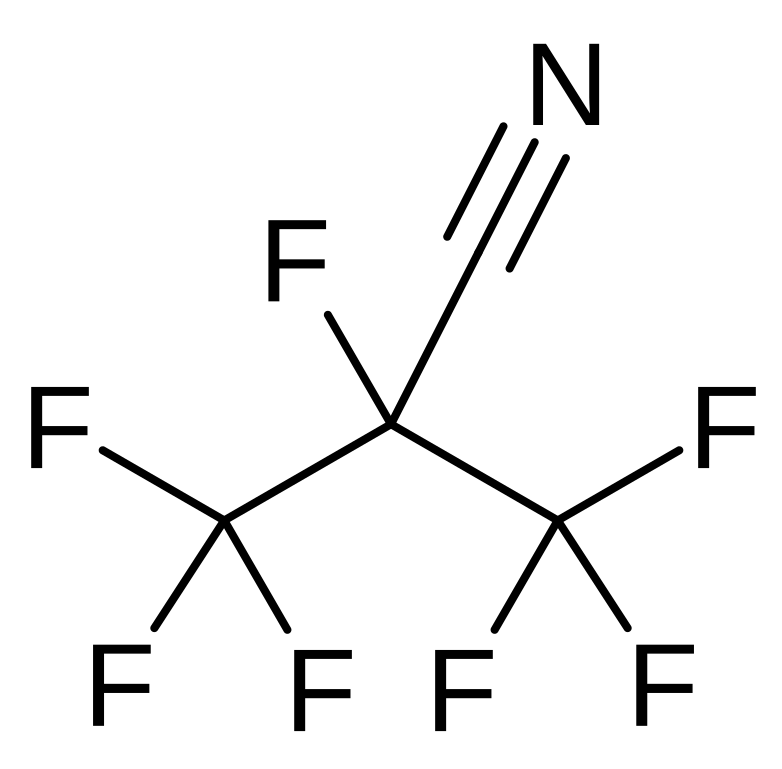
C4F7N, also known as 2,3,3,3-tetrafluoro-2-(trifluoromethyl)propanenitrile, is a fluorocarbon compound with a unique molecular structure that underpins its utility in demanding applications. Below are its key properties:
-
Molecular Structure: C4F7N consists of four carbon atoms, seven fluorine atoms, and one nitrogen atom, with a nitrile group (-C≡N) that enhances its chemical stability and dielectric strength.
-
Physical State: At room temperature, C4F7N is a colorless, odorless gas, though it can be liquefied under moderate pressure for storage and transport.
-
Boiling Point: Approximately -4.7°C at standard pressure, making it suitable for gaseous applications in electrical systems.
-
Dielectric Strength: C4F7N exhibits a dielectric strength about twice that of SF6 when mixed with buffer gases like CO2 or N2, enabling effective insulation in high-voltage equipment.
-
Global Warming Potential (GWP): With a GWP of ~2,100, C4F7N is significantly less environmentally damaging than SF6, aligning with global sustainability goals.
-
Thermal Stability: C4F7N remains stable at high temperatures, making it ideal for arc quenching in circuit breakers, where electrical arcs generate intense heat.
-
Toxicity: While less toxic than some fluorocarbons, C4F7N requires careful handling due to potential decomposition products under extreme conditions.
These properties make C4F7N a compelling alternative to SF6, balancing performance with environmental responsibility.
Production Methods of C4F7N
Producing high-purity C4F7N requires advanced chemical synthesis techniques to ensure consistency and safety. The primary methods include:
1. Fluorination of Organic Precursors
C4F7N is typically synthesized through the fluorination of organic nitriles or related compounds. One common approach involves reacting perfluorinated precursors with nitrogen-containing reagents under controlled conditions:
[ \ce{(CF3)2C=CF2 + CN- \rightarrow (CF3)2CFC≡N} ]
This process requires catalysts and precise temperature control to achieve high yields and purity levels above 99.9%, critical for electrical and semiconductor applications.
2. Electrochemical Fluorination
Electrochemical fluorination (ECF) is another method, where organic compounds are fluorinated in an electrolytic cell using anhydrous hydrogen fluoride. This technique is effective for producing fluorocarbons like C4F7N but is energy-intensive and requires rigorous purification to remove impurities.
3. Advanced Purification Techniques
Post-synthesis, C4F7N undergoes fractional distillation and adsorption processes to achieve ultra-high purity. Companies like Asia Isotope International have developed proprietary purification methods to meet the stringent requirements of high-voltage and semiconductor industries.
The choice of production method depends on factors like cost, scale, and application-specific purity needs. Advances in green chemistry are also exploring more sustainable synthesis routes to reduce energy consumption and waste.
Applications in Electrical Insulation and Arc Quenching
C4F7N’s primary application lies in replacing SF6 in high-voltage electrical systems, where its dielectric properties and lower GWP offer significant advantages. Below are its key uses:
1. High-Voltage Gas-Insulated Switchgear (GIS)
Gas-insulated switchgear relies on dielectric gases to prevent electrical discharges in compact, high-voltage systems. C4F7N, often mixed with buffer gases like CO2 or N2, provides insulation performance comparable to SF6 while reducing environmental impact. For example, a 20% C4F7N/80% CO2 mixture achieves dielectric strength close to pure SF6, making it suitable for GIS in power grids and substations.
2. Circuit Breakers and Arc Quenching
In circuit breakers, C4F7N excels at quenching electrical arcs—plasma discharges that occur when circuits are interrupted. Its thermal stability and ability to rapidly cool arcs ensure reliable operation in high-voltage environments, such as those in renewable energy systems like wind and solar farms.
3. Gas-Insulated Transmission Lines (GIL)
C4F7N is increasingly used in gas-insulated transmission lines, where its high dielectric strength enables efficient power transmission over long distances. This application is critical for modernizing aging electrical grids to support renewable energy integration.
4. Environmental Benefits
By replacing SF6, which has a 3,200-year atmospheric lifetime, C4F7N reduces greenhouse gas emissions in electrical systems. Its shorter atmospheric lifetime (~30 years) and lower GWP make it a cornerstone of sustainable power infrastructure, aligning with regulations like the EU’s F-Gas Regulation and the Kyoto Protocol.
Applications Beyond Electrical Systems
While C4F7N’s primary role is in electrical insulation, its properties enable applications in other high-tech fields:
1. Semiconductor Manufacturing
In semiconductor fabrication, C4F7N is used as an etching gas in plasma etching processes to create intricate patterns on silicon wafers. Its high purity and chemical stability ensure precise etching, critical for producing microchips and integrated circuits. Compared to traditional fluorocarbons like CF4, C4F7N offers improved selectivity and reduced environmental impact.
2. Specialty Chemical Synthesis
C4F7N serves as a building block in synthesizing fluorinated compounds for pharmaceuticals and agrochemicals. Its nitrile group enables reactions like nucleophilic additions, producing intermediates for high-value molecules used in drug development.
3. Refrigeration and Cooling Systems
Although less common, C4F7N is being explored as a component in low-GWP refrigerant blends, leveraging its thermal stability and low toxicity. This application aligns with the global phase-down of high-GWP hydrofluorocarbons (HFCs) under the Kigali Amendment.
Safety and Handling Considerations
C4F7N’s chemical stability reduces some risks associated with reactive gases, but its handling requires careful attention:
-
Storage: C4F7N is stored in high-pressure cylinders with corrosion-resistant materials (e.g., stainless steel) to prevent degradation. Low-temperature storage minimizes pressure buildup.
-
Handling: Facilities must use leak-detection systems and ventilation to manage potential releases. Decomposition products under high-voltage conditions (e.g., fluorinated byproducts) require monitoring.
-
Personal Protective Equipment (PPE): Workers should wear respiratory protection and chemical-resistant gloves to avoid exposure to potential decomposition products.
-
Regulatory Compliance: Handling must comply with international standards like IEC 62271-4 for gas-insulated equipment and local environmental regulations.
A 2018 incident involving SF6 leakage in a European substation underscored the need for robust safety protocols for dielectric gases, highlighting the importance of adopting safer alternatives like C4F7N.
Recent Advances in C4F7N Research
Research into C4F7N has accelerated, driven by the need for sustainable alternatives to SF6. Key developments include:
-
Mixture Optimization: Studies, such as those published in IEEE Transactions on Dielectrics and Electrical Insulation (2022), have optimized C4F7N/CO2 mixtures to achieve dielectric performance comparable to SF6 while minimizing GWP. A 10-20% C4F7N blend is now standard in many GIS applications.
-
Decomposition Studies: Research in Journal of Fluorine Chemistry (2023) has analyzed C4F7N’s decomposition under arc conditions, identifying safe byproducts and improving equipment design.
-
Scalable Production: Companies like 3M and Asia Isotope International have scaled up C4F7N production, achieving purities above 99.99% for semiconductor and electrical applications.
These advances underscore C4F7N’s potential to replace SF6 across multiple industries while meeting stringent environmental standards.
Market Trends and Economic Significance
The global market for dielectric gases is growing, driven by the transition to sustainable energy systems and stricter environmental regulations. The specialty gas market, including C4F7N, was valued at USD 2.8 billion in 2023 and is projected to grow at a CAGR of 7.5% through 2030, according to industry reports. C4F7N’s adoption in high-voltage equipment and semiconductors is a key driver of this growth.
Europe leads the adoption of C4F7N due to stringent F-Gas regulations, with countries like Germany and France integrating it into renewable energy projects. The Asia-Pacific region, particularly China, is a major producer, leveraging its chemical manufacturing infrastructure to supply high-purity C4F7N globally.
Challenges and Future Directions
Despite its promise, C4F7N faces challenges that must be addressed to ensure widespread adoption:
-
Cost: C4F7N is more expensive to produce than SF6, requiring economies of scale to reduce costs. Advances in synthesis and purification are critical to improving affordability.
-
Compatibility: Existing SF6-based equipment may require retrofitting to accommodate C4F7N mixtures, posing logistical challenges for utilities.
-
Regulatory Evolution: While C4F7N’s GWP is low compared to SF6, future regulations may demand even lower-GWP alternatives, necessitating ongoing innovation.
Looking ahead, C4F7N’s role in green energy and electronics is set to expand. Its use in next-generation GIS and circuit breakers will support the global transition to renewable energy, while its applications in semiconductor manufacturing align with the growth of AI and IoT technologies. Research into novel fluorocarbon blends and recycling methods will further enhance C4F7N’s sustainability.
Conclusion
Perfluoroisobutyronitrile (C4F7N) represents a paradigm shift in the specialty gas industry, offering a low-GWP alternative to SF6 for electrical insulation and arc quenching. Its exceptional dielectric strength, thermal stability, and versatility in applications like semiconductor etching and chemical synthesis make it a cornerstone of sustainable, high-performance technologies. Drawing on over 30 years of expertise in specialty gases, I can affirm that C4F7N’s combination of environmental benefits and technical prowess positions it as a leader in the transition to greener industrial practices. As research and adoption accelerate, C4F7N will play a pivotal role in shaping the future of electrical and chemical industries.
Sources:
-
IEEE Transactions on Dielectrics and Electrical Insulation, 2022, “Dielectric Properties of C4F7N/CO2 Mixtures”
-
Journal of Fluorine Chemistry, 2023, “Decomposition Pathways of Heptafluoroisobutyronitrile”
-
Industry reports on specialty gas markets, 2023–2030 projections
-
EU F-Gas Regulation (Regulation (EU) No 517/2014)
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)