C4H4 Vinyl Acetylene: The Ultimate Specialty Gas for High-Performance Chemical Manufacturing
BY Tao, Published August 27, 2025
Introduction to Vinyl Acetylene (C4H4)
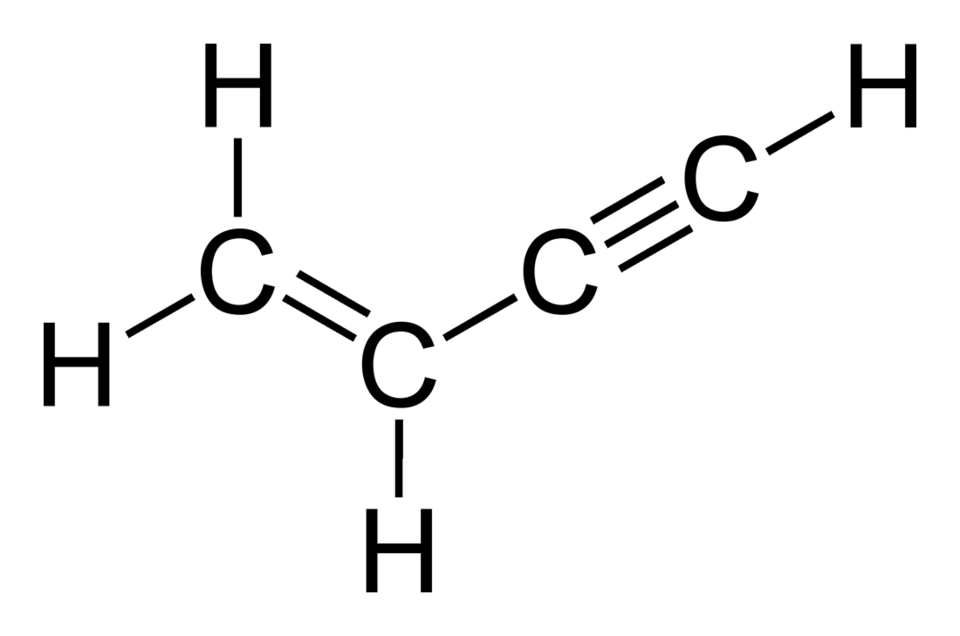
Vinyl acetylene, chemically known as 1-buten-3-yne (C4H4), is a unique hydrocarbon that combines the structural features of both an alkene (double bond) and an alkyne (triple bond), making it the simplest enyne. This colorless gas, with the molecular formula H2C=CH−C≡CH, has a rich history in the chemical industry and continues to play a pivotal role in high-performance chemical manufacturing. Its versatility as a chemical intermediate stems from its reactive carbon-carbon bonds, which enable a wide range of synthetic transformations. In this article, we explore the properties, production methods, applications, and safety considerations of vinyl acetylene, underscoring its significance as a specialty gas in modern industrial processes.
Vinyl acetylene’s importance lies in its ability to serve as a building block for synthesizing complex organic compounds, such as neoprene, pharmaceuticals, and advanced materials. Its high reactivity, while a boon for chemical synthesis, also necessitates stringent handling protocols due to its flammability and potential for auto-detonation under certain conditions. As a seasoned researcher with over two decades of experience in specialty gases, I aim to demystify this fascinating compound and highlight its critical role in advancing chemical manufacturing.
Chemical and Physical Properties of Vinyl Acetylene
Vinyl acetylene is a highly reactive molecule due to its conjugated system of double and triple bonds. Below are its key chemical and physical properties, which underpin its utility in industrial applications:
-
Molecular Structure: Vinyl acetylene consists of four carbon atoms and four hydrogen atoms, arranged as H2C=CH−C≡CH. The presence of a double bond (alkene) and a triple bond (alkyne) in close proximity creates a conjugated system, enhancing its reactivity.
-
Physical State: At room temperature, vinyl acetylene is a colorless gas, though it can be liquefied under pressure or dissolved in solvents like p-xylene for safer handling.
-
Boiling Point: Approximately 5°C at standard pressure, making it volatile and requiring careful storage.
-
Density: As a gas, it is slightly lighter than air, with a density of about 0.908 g/L.
-
Reactivity: The conjugated bonds make vinyl acetylene highly reactive, particularly in addition reactions and polymerization processes. It can undergo dimerization, addition with hydrogen halides, or cyclization to form aromatic compounds.
-
Stability: Vinyl acetylene is unstable in high concentrations (>30 mole percent) and under elevated pressures, where it may auto-detonate without oxygen. This property demands rigorous safety measures in industrial settings.
These properties make vinyl acetylene a versatile yet challenging compound to work with, requiring expertise in handling specialty gases to harness its potential safely.
Production Methods of Vinyl Acetylene
The production of vinyl acetylene has evolved over time, with several methods developed to meet industrial demands. Below are the primary synthetic routes:
1. Dimerization of Acetylene
One of the most common methods for producing vinyl acetylene is the dimerization of acetylene (C2H2) in the presence of a catalyst, typically copper(I) chloride. This reaction can be represented as:
[ 2C_2H_2 \xrightarrow{\ce{CuCl}} H_2C=CH-C≡CH ]
This process is efficient and leverages the abundance of acetylene, a widely available petrochemical. However, it requires precise control to prevent side reactions, such as further polymerization into polyacetylene.
2. Dehydrohalogenation of 1,3-Dichloro-2-Butene
Another route involves the dehydrohalogenation of 1,3-dichloro-2-butene, where the elimination of hydrogen chloride yields vinyl acetylene. This method was historically significant in early industrial processes:
[ \ce{CH3-CHCl-CH=CH2 \rightarrow H2C=CH-C≡CH + HCl} ]
This approach is less common today due to the availability of more cost-effective acetylene-based methods.
3. Other Synthetic Pathways
Vinyl acetylene can also be produced through the dehydrogenation of 1,3-butadiene, though this method is less prevalent due to lower yields. Additionally, modern research explores plasma chemistry and other advanced techniques to produce vinyl acetylene in controlled environments, particularly for high-purity applications.
Each method has its advantages and challenges, with the choice depending on factors like cost, scale, and desired purity. For high-performance chemical manufacturing, high-purity vinyl acetylene (e.g., 98% or higher) is often required, necessitating advanced purification techniques such as distillation or solvent dissolution.
Applications in High-Performance Chemical Manufacturing
Vinyl acetylene’s unique chemical structure makes it a cornerstone in the synthesis of various high-value products. Below, we explore its key applications, emphasizing its role as a specialty gas.
1. Neoprene Production
Vinyl acetylene is a critical intermediate in the production of neoprene, a synthetic rubber known for its resistance to oil, solvents, and weathering. The process involves the dimerization of acetylene to form vinyl acetylene, followed by reaction with hydrogen chloride to produce 4-chloro-1,2-butadiene, which is further processed into chloroprene, the monomer for neoprene. This application was historically significant and remains relevant in industries requiring durable, chemical-resistant materials.
2. Synthesis of Fine Chemicals and Pharmaceuticals
Vinyl acetylene serves as a versatile building block in organic synthesis, particularly for fine chemicals and pharmaceuticals. Its reactive double and triple bonds allow it to participate in addition reactions, cycloadditions, and polymerization processes. For instance, vinyl acetylene is used in the synthesis of:
-
Polycyclic Aromatic Hydrocarbons (PAHs): Vinyl acetylene reacts with PAH radicals to form larger aromatic structures, such as phenanthrene and anthracene, which are precursors to advanced materials and dyes.
-
Specialty Polymers: It is used to produce polymers with unique properties, such as high thermal stability and conductivity, for applications in electronics and coatings.
-
Pharmaceutical Intermediates: Vinyl acetylene’s reactivity enables the synthesis of complex molecules used in drug development, particularly those requiring conjugated systems.
3. Advanced Materials for Electronics
The electronics industry benefits from vinyl acetylene’s role in producing high-purity materials for coatings, adhesives, and conductive polymers. For example, its use in chemical vapor deposition (CVD) processes helps create thin films for semiconductor manufacturing, where high purity and precise control are paramount.
4. Contribution to PAH Growth in Combustion
Vinyl acetylene plays a significant role in the formation of polycyclic aromatic hydrocarbons in combustion environments, such as flames and chemical reactors. Its abundance in ethylene, benzene, and toluene flames makes it a key species in the growth of soot precursors, which has implications for both environmental science and material synthesis. Computational studies have shown that vinyl acetylene reacts with naphthyl radicals to form phenanthrene and other PAHs, highlighting its importance in carbon chemistry.
5. Other Organic Syntheses
Beyond neoprene and PAHs, vinyl acetylene is used in the synthesis of various organic compounds, including acetylenic alcohols (used in vitamin production) and specialty monomers for synthetic rubbers. Its versatility as a chemical intermediate makes it indispensable in diverse industrial applications.
Safety and Handling Considerations
While vinyl acetylene’s reactivity makes it valuable, it also poses significant safety challenges. Its potential for auto-detonation at concentrations above 30 mole percent and under elevated pressures necessitates strict handling protocols. Below are key safety considerations:
-
Storage: Vinyl acetylene is often stored as a solution (e.g., 50% in p-xylene) to reduce its volatility and risk of explosion. High-pressure cylinders must be equipped with pressure-relief devices and stored in well-ventilated areas away from ignition sources.
-
Handling: Operators must use explosion-proof equipment and avoid conditions that could lead to high concentrations of the gas. Inert gas purging (e.g., with nitrogen) is recommended during transfer operations.
-
Personal Protective Equipment (PPE): Workers handling vinyl acetylene should wear flame-resistant clothing, gloves, and eye protection to mitigate risks from potential leaks or reactions.
-
Emergency Protocols: Facilities must have robust emergency response plans, including fire suppression systems and evacuation procedures, due to the gas’s flammability and explosive potential.
A notable example of the risks associated with vinyl acetylene is the 1969 explosion at a Union Carbide plant in Texas City, caused by improper handling of C4 hydrocarbons. This incident underscores the importance of adhering to safety standards in chemical manufacturing.
Advances in Vinyl Acetylene Research
Recent research has expanded our understanding of vinyl acetylene’s role in chemical manufacturing and combustion chemistry. Key advancements include:
-
Computational Studies: Density Functional Theory (DFT) and Coupled Cluster methods have been used to study the reaction of vinyl acetylene with PAH radicals, revealing pathways for forming phenanthrene and other complex hydrocarbons. These studies inform the development of cleaner combustion processes and advanced materials.
-
High-Purity Production: Companies like Shanghai Wechem Chemical Co., Ltd. and Asia Isotope International have developed state-of-the-art facilities to produce high-purity vinyl acetylene (up to 98%) for specialized applications in electronics and pharmaceuticals.
-
Environmental Implications: Research into vinyl acetylene’s role in soot formation has implications for reducing emissions in combustion processes, aligning with global efforts to mitigate air pollution.
These advancements highlight the ongoing relevance of vinyl acetylene in cutting-edge research and its potential to address modern challenges in chemical manufacturing.
Market Trends and Economic Significance
The global market for specialty gases, including vinyl acetylene, is growing due to increasing demand for high-performance materials in electronics, pharmaceuticals, and energy sectors. While vinyl acetylene is a niche product compared to acetylene or ethylene, its role in high-value applications ensures its economic significance. For instance, the acetylene gas market, which includes vinyl acetylene derivatives, was valued at USD 5.76 billion in 2023 and is projected to reach USD 7.67 billion by 2032, driven by applications in chemical synthesis and advanced materials.
Asia-Pacific, particularly China, dominates the production and consumption of vinyl acetylene due to its robust chemical industry and access to low-cost raw materials like acetylene. Companies in this region are investing in advanced production technologies to meet the growing demand for high-purity specialty gases.
Future Prospects and Challenges
The future of vinyl acetylene in chemical manufacturing is promising but not without challenges. Its high reactivity and safety concerns necessitate ongoing improvements in production, storage, and handling technologies. Additionally, competition from alternative feedstocks, such as ethylene-based processes, poses a challenge to vinyl acetylene’s market share in certain applications, like vinyl acetate production.
However, opportunities abound in emerging fields like nanotechnology and green chemistry. For example, vinyl acetylene’s role in synthesizing conductive polymers and carbon-based nanomaterials could position it as a key player in next-generation electronics and energy storage systems. Furthermore, advancements in catalytic processes may enhance the efficiency and sustainability of vinyl acetylene production, reducing its environmental footprint.
Conclusion
Vinyl acetylene (C4H4) is a cornerstone of high-performance chemical manufacturing, offering unparalleled versatility as a chemical intermediate. Its unique structure enables the synthesis of critical materials like neoprene, PAHs, and specialty polymers, while its reactivity demands careful handling to ensure safety. As a specialty gas, vinyl acetylene continues to drive innovation in industries ranging from electronics to pharmaceuticals, supported by advancements in production and computational research.
With over two decades of experience in specialty gases, I can attest to the enduring importance of vinyl acetylene in shaping the future of chemical manufacturing. Its role in creating high-value products underscores the need for continued investment in safe handling practices and cutting-edge research to unlock its full potential.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)