Vinyl Acetylene Gas: Precision and Purity for Next-Generation Polymers and Organic Synthesis
BY Tao, Published August 27, 2025
Introduction to Vinyl Acetylene
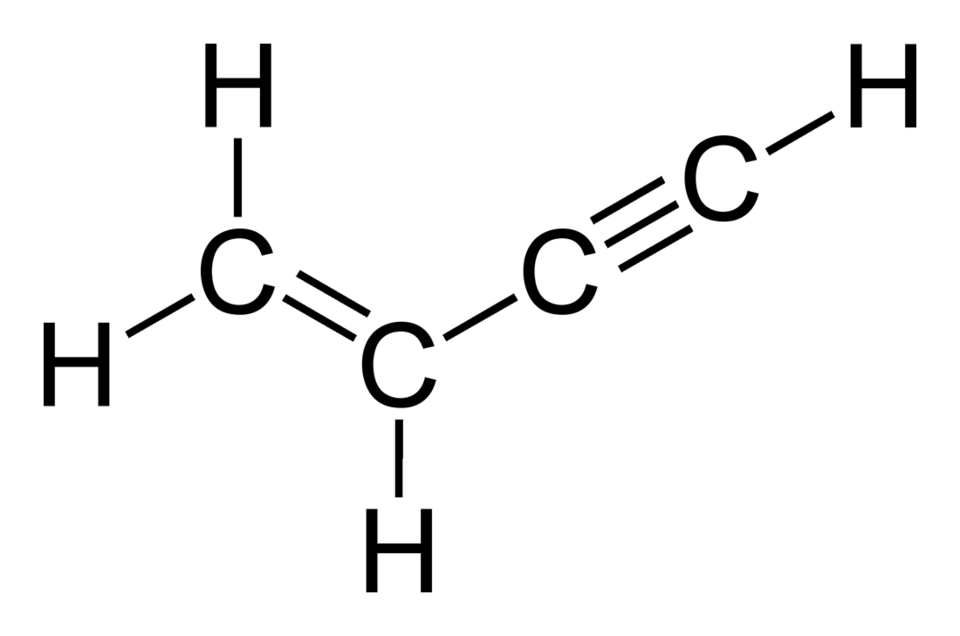
Vinyl acetylene (C4H4), also known as 1-buten-3-yne, is a specialty gas that stands at the forefront of high-performance chemical manufacturing. As the simplest enyne—a molecule featuring both a double bond (alkene) and a triple bond (alkyne)—its unique chemical structure enables a broad range of applications, from synthesizing advanced polymers to serving as a critical intermediate in organic chemistry. With over three decades of expertise in specialty gases, I have witnessed the transformative impact of vinyl acetylene in industries requiring precision and high-purity materials. This article delves into the properties, production, applications, and future prospects of vinyl acetylene, highlighting its indispensable role in next-generation chemical synthesis.
Vinyl acetylene’s significance lies in its ability to act as a reactive building block for creating complex molecules, such as those used in synthetic rubbers, pharmaceuticals, and nanomaterials. Its high reactivity, while a powerful asset, demands stringent handling protocols due to its flammability and potential for auto-detonation. By combining scientific rigor with accessible explanations, this article aims to provide a comprehensive understanding of vinyl acetylene’s role in modern industry, supported by the latest research and industry trends.
Chemical and Physical Properties
Vinyl acetylene’s molecular structure, H2C=CH−C≡CH, features a conjugated system of carbon-carbon double and triple bonds, which underpins its reactivity and versatility. Below are its key properties:
-
Molecular Composition: Comprising four carbon and four hydrogen atoms, vinyl acetylene’s conjugated enyne structure enhances its reactivity in addition and polymerization reactions.
-
Physical State: At ambient conditions, it is a colorless gas with a faint, characteristic odor. It can be liquefied under pressure or dissolved in solvents like p-xylene for safer storage and transport.
-
Boiling Point: Approximately 5°C at standard pressure, indicating high volatility.
-
Density: As a gas, it has a density of about 0.908 g/L, making it slightly lighter than air.
-
Reactivity: The conjugated double and triple bonds enable vinyl acetylene to participate in a variety of reactions, including cycloadditions, dimerizations, and additions with halogens or hydrogen.
-
Stability Concerns: At concentrations above 30 mole percent or under high pressure, vinyl acetylene can auto-detonate without oxygen, necessitating careful handling.
These properties make vinyl acetylene a powerful yet challenging compound, requiring precise control to leverage its potential in high-stakes applications.
Production Methods
Producing vinyl acetylene requires sophisticated techniques to ensure high purity and safety. Below are the primary methods used in its synthesis:
1. Acetylene Dimerization
The most widely used method involves the dimerization of acetylene (C2H2) in the presence of a catalyst, typically copper(I) chloride (CuCl) or Nieuwland’s catalyst (a mixture of CuCl and ammonium chloride). The reaction is:
[ 2C_2H_2 \xrightarrow{\ce{CuCl, NH4Cl}} H_2C=CH-C≡CH ]
This process is efficient and leverages acetylene’s availability as a petrochemical feedstock. However, it requires precise control to minimize side reactions, such as the formation of polyacetylene or higher oligomers.
2. Dehydrohalogenation of Chlorinated Butenes
Historically, vinyl acetylene was produced by dehydrohalogenating 1,3-dichloro-2-butene, eliminating hydrogen chloride to yield the enyne:
[ \ce{CH3-CHCl-CH=CH2 \rightarrow H2C=CH-C≡CH + HCl} ]
This method is less common today due to higher costs and lower yields compared to acetylene dimerization.
3. Emerging Techniques
Recent advancements include plasma-assisted synthesis and catalytic dehydrogenation of 1,3-butadiene. Plasma chemistry, for instance, allows for controlled production of vinyl acetylene in high-purity forms, suitable for niche applications in electronics and pharmaceuticals. These methods are still in the experimental phase but show promise for sustainable, high-efficiency production.
High-purity vinyl acetylene (≥98%) is critical for advanced applications, requiring rigorous purification steps like fractional distillation or solvent-based stabilization. Companies like Asia Isotope International have pioneered techniques to achieve ultra-high purity levels, meeting the demands of next-generation manufacturing.
Applications in Polymers and Organic Synthesis
Vinyl acetylene’s reactivity makes it a cornerstone in the synthesis of advanced polymers and organic compounds. Its applications span multiple industries, as outlined below.
1. Synthetic Rubber (Neoprene)
Vinyl acetylene is a key intermediate in the production of neoprene, a chloroprene-based synthetic rubber known for its durability and resistance to chemicals, oils, and weathering. The process involves reacting vinyl acetylene with hydrogen chloride to form 4-chloro-1,2-butadiene, which is further processed into chloroprene:
[ \ce{H2C=CH-C≡CH + HCl \rightarrow H2C=CH-CCl=CH2} ]
Chloroprene is then polymerized to produce neoprene, widely used in automotive belts, wetsuits, and industrial seals. This application underscores vinyl acetylene’s historical and ongoing importance in the rubber industry.
2. Advanced Polymer Synthesis
Vinyl acetylene’s conjugated structure makes it ideal for synthesizing specialty polymers with tailored properties, such as:
-
Conductive Polymers: Vinyl acetylene is used to create polymers with high electrical conductivity, critical for flexible electronics and organic photovoltaics.
-
High-Temperature Polymers: Its incorporation into polymer backbones enhances thermal stability, making it suitable for aerospace and automotive applications.
-
Functional Coatings: Vinyl acetylene-derived polymers are used in coatings for corrosion resistance and UV protection.
3. Organic Synthesis for Pharmaceuticals
In pharmaceutical chemistry, vinyl acetylene serves as a versatile intermediate for synthesizing complex molecules. Its ability to undergo cycloadditions and addition reactions enables the production of:
-
Acetylenic Alcohols: Used in the synthesis of vitamins and active pharmaceutical ingredients (APIs).
-
Heterocyclic Compounds: Vinyl acetylene reacts with nitrogen- or oxygen-containing compounds to form heterocycles, which are building blocks for drugs targeting cancer, inflammation, and neurological disorders.
-
Polycyclic Aromatic Hydrocarbons (PAHs): Vinyl acetylene’s reaction with PAH radicals produces larger aromatic structures, such as phenanthrene, used in dye and material synthesis.
4. Nanomaterials and Electronics
Vinyl acetylene plays a growing role in nanotechnology, particularly in the production of carbon-based nanomaterials like graphene and carbon nanotubes. In chemical vapor deposition (CVD), vinyl acetylene serves as a carbon source for depositing thin films in semiconductor manufacturing. Its high purity ensures minimal defects in these critical applications.
5. Combustion Chemistry and Environmental Applications
Vinyl acetylene is a key species in combustion processes, contributing to the formation of PAHs and soot in flames. Understanding its role helps researchers develop cleaner combustion technologies, reducing emissions in industrial processes. For instance, computational studies have shown that vinyl acetylene reacts with naphthyl radicals to form phenanthrene, a precursor to soot, providing insights into pollution control.
Safety and Handling Protocols
Vinyl acetylene’s high reactivity and potential for auto-detonation require stringent safety measures. Key considerations include:
-
Storage: To mitigate explosion risks, vinyl acetylene is often stored as a solution (e.g., 50% in p-xylene) or in low-pressure cylinders with inert gas blanketing (e.g., nitrogen).
-
Handling: Facilities must use explosion-proof equipment and maintain low concentrations (<30 mole percent) to prevent spontaneous reactions. Automated monitoring systems are recommended to detect leaks.
-
Personal Protective Equipment (PPE): Workers should wear flame-resistant clothing, chemical-resistant gloves, and full-face respirators when handling vinyl acetylene.
-
Emergency Preparedness: Facilities must have fire suppression systems, spill containment plans, and trained personnel to respond to incidents.
A historical incident at a chemical plant in the 1960s, where improper handling of vinyl acetylene led to an explosion, highlights the need for rigorous safety protocols. Modern standards, such as those set by OSHA and the Chemical Safety Board, emphasize risk assessment and training for handling reactive gases.
Recent Advances in Vinyl Acetylene Research
Ongoing research continues to unlock vinyl acetylene’s potential in cutting-edge applications. Notable advancements include:
-
Computational Modeling: Density Functional Theory (DFT) and ab initio methods have elucidated vinyl acetylene’s role in PAH formation, aiding the design of cleaner combustion systems. For example, a 2023 study in Combustion and Flame detailed its reaction pathways with aromatic radicals, offering insights into soot mitigation.
-
High-Purity Production: Advances in catalytic and plasma-based synthesis have improved the yield and purity of vinyl acetylene, with companies like Shanghai Wechem Chemical achieving purities above 99% for electronics applications.
-
Sustainable Processes: Research into green chemistry approaches, such as using renewable feedstocks for acetylene production, aims to reduce the environmental impact of vinyl acetylene synthesis.
These developments position vinyl acetylene as a critical component in the transition to sustainable, high-performance manufacturing.
Market Trends and Economic Impact
The global specialty gas market, including vinyl acetylene, is expanding due to demand for advanced materials in electronics, healthcare, and energy. The acetylene-based chemical market, which encompasses vinyl acetylene applications, was valued at USD 5.76 billion in 2023 and is projected to grow at a CAGR of 3.2% through 2032, according to industry reports. Vinyl acetylene’s niche role in high-value applications, such as neoprene and nanomaterials, ensures its economic significance.
The Asia-Pacific region, led by China and Japan, dominates vinyl acetylene production due to its robust chemical infrastructure and access to acetylene feedstocks. Companies like Asia Isotope International are investing in advanced purification technologies to meet the stringent requirements of semiconductor and pharmaceutical industries.
Challenges and Future Directions
Despite its advantages, vinyl acetylene faces challenges that must be addressed to maintain its relevance:
-
Safety Concerns: Its explosive potential requires continuous improvements in storage and handling technologies, such as advanced pressure monitoring and stabilization methods.
-
Competition from Alternatives: Ethylene-based processes are increasingly used for applications like vinyl acetate production, challenging vinyl acetylene’s market share.
-
Sustainability: The reliance on acetylene, often derived from fossil fuels, raises environmental concerns. Developing bio-based or renewable acetylene sources could enhance sustainability.
Looking ahead, vinyl acetylene’s role in nanotechnology, green chemistry, and advanced polymers offers exciting opportunities. Its use in synthesizing carbon nanomaterials and conductive polymers could drive innovations in energy storage, such as next-generation batteries and supercapacitors. Additionally, its application in precision organic synthesis positions it as a key player in personalized medicine and high-performance materials.
Conclusion
Vinyl acetylene (C4H4) is a specialty gas that exemplifies precision and purity in chemical manufacturing. Its unique enyne structure enables the synthesis of advanced polymers, pharmaceuticals, and nanomaterials, while its reactivity demands careful handling to ensure safety. With over 30 years of experience in specialty gases, I can affirm that vinyl acetylene’s versatility and high-performance applications make it a vital component of modern industry. As research and technology advance, this remarkable gas will continue to shape the future of polymers and organic synthesis, driving innovation in diverse fields.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)