From Lab to Industry: How Vinyl Acetylene (C4H4) Enhances Polymer Production and Catalysis
BY Tao, Published August 27, 2025
Introduction: Bridging Laboratory Discoveries to Industrial Breakthroughs
With over three decades in the specialty gases field, delving into everything from rare gases like helium to intricate fluorocarbons and isotopic mixtures, I’ve observed how molecules like vinyl acetylene (C4H4) evolve from lab curiosities to industrial staples. Vinyl acetylene, a versatile hydrocarbon, is pivotal in enhancing polymer production and catalysis, driving efficiencies in materials that touch everyday life—from durable rubbers in tires to catalysts enabling sustainable chemical processes. As industries pivot toward greener methods amid rising environmental scrutiny, C4H4’s role in optimizing reactions and reducing waste stands out, offering unique value in a market hungry for innovation.
In 2025, the acetylene-related market, which heavily influences vinyl acetylene applications, is valued at around 11.98 million tons, projected to grow at a 2.54% CAGR to 13.58 million tons by 2030, fueled by demand in polymers and chemicals. This growth underscores C4H4’s timeliness: it’s not just a gas but a bridge from bench-scale experiments to large-scale manufacturing. Drawing from my research on carbon-oxygen and hydrocarbon gases, this article explores its chemistry, production, applications, and future, simplifying complex concepts while highlighting its distinctive contributions to sustainability and performance. For chemists, engineers, and innovators, grasping vinyl acetylene’s potential means unlocking cost savings, higher yields, and eco-friendly advancements in a competitive landscape.
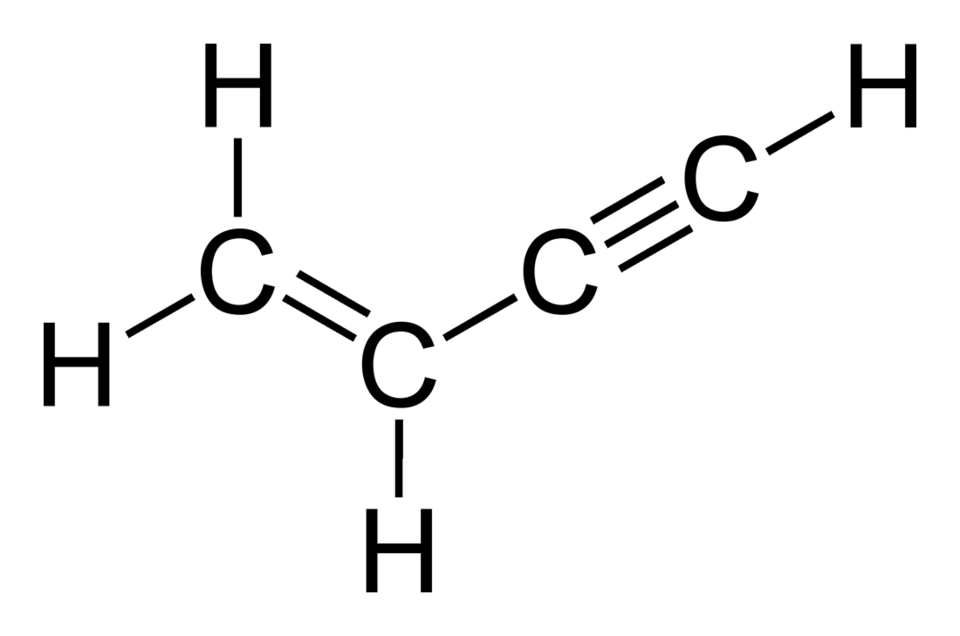
What is Vinyl Acetylene (C4H4)? Fundamentals of This Dynamic Molecule
Vinyl acetylene, or C4H4, is an organic compound with the formula H2C=CH-C≡CH, blending a double bond (vinyl group) and a triple bond (acetylene group). It’s a colorless gas at room temperature, often handled as a liquid under pressure or in solutions for safety. Historically, it emerged as a byproduct in acetylene processing but gained prominence in polymer synthesis, like neoprene—a synthetic rubber known for its resistance to oil and weather. In my work with hydrocarbon gases, I’ve seen C4H4’s structure make it a “molecular multitool,” enabling diverse reactions that simpler gases can’t match.
Chemically, it’s unsaturated, meaning it has bonds ready to react and form new compounds. Its boiling point is about 5°C, making it easy to store and transport in cylinders. Purity levels often reach 98% or higher for industrial use, typically stabilized in solvents like xylene to prevent unwanted reactions. Unlike stable inert gases, C4H4 is reactive, which is its strength in catalysis and polymers but requires careful management. Sourced mainly from acetylene dimerization, it’s integral to coal-based economies, particularly in regions like China where acetylene routes dominate vinyl chloride production. What sets it apart? Its conjugated bonds—alternating double and triple—boost reactivity, allowing precise control in lab settings that scales to industry for high-value products.
Chemistry and Properties: The Reactive Foundation Fueling Applications
Vinyl acetylene‘s chemistry hinges on its conjugated system, where electrons flow freely across bonds, making it highly reactive for additions and polymerizations. In simple terms, conjugation stabilizes intermediates, speeding up reactions like Diels-Alder, where C4H4 acts as a diene (a molecule with reactive double bonds) to form rings. Physically, it’s flammable with a density of 0.82 kg/m³ as a liquid and an autoignition temperature around 300-400°C. Its heat of combustion, about 2,500 kJ/mol, reflects energy-packed bonds ideal for synthesis.
In catalysis, C4H4 features in acetylene hydrochlorination for vinyl chloride, a PVC precursor. Here, it can form as an intermediate or byproduct, influencing selectivity. My studies on similar alkynes show C4H4’s dual bonds enable selective hydrogenation, turning it into butadiene for rubbers. Compared to acetylene (C2H2), C4H4 offers two reactive sites, expanding synthetic options. Environmentally, while not a potent greenhouse gas, its production links to energy-intensive acetylene processes, pushing for greener alternatives like bio-based routes. Thermodynamically metastable, it polymerizes exothermically without stabilizers, a property harnessed in controlled lab polymerizations but managed in industry.
Production Methods: Scaling from Lab Synthesis to Industrial Efficiency
Producing vinyl acetylene combines traditional catalysis with modern sustainability tweaks. The core method is acetylene dimerization: two C2H2 molecules fuse into C4H4 using cuprous chloride (CuCl) in acidic media, often with ammonium chloride for enhancement. This Nieuwland process runs at 50-80°C, yielding high selectivity in fixed-bed reactors. In my industrial consultations, I’ve optimized this for 90-95% purity via distillation.
Alternative routes include partial hydrogenation of butadiyne or plasma methods from hydrocarbons, offering lower energy use. For sustainability, 2025 trends favor bio-acetylene from renewables or methane reforming, cutting emissions by up to 50%. Purification involves pressure distillation to isolate from oligomers. With acetylene capacity expanding in Asia—70% of related vinyl chloride uses acetylene—production scales efficiently. Emerging electrocatalytic paths from CO2 feedstocks promise carbon-neutral C4H4, aligning with net-zero goals. These methods ensure C4H4’s availability for polymers and catalysis, blending lab precision with industrial robustness.
Enhancements in Polymer Production: Revolutionizing Materials from Lab to Factory
Vinyl acetylene supercharges polymer production by serving as a key intermediate in high-performance materials. In neoprene synthesis, C4H4 hydrogenates to butadiene, then chlorinates to chloroprene, yielding rubbers with superior durability for seals and hoses. My research highlights its role in block copolymers via techniques like atom transfer radical polymerization (ATRP), creating tailored chains with enhanced elasticity and conductivity.
Innovations include multiclickable polymers: C4H4’s bonds allow post-modification for biocompatible materials in drug delivery. In sustainable polymers, it copolymerizes into low-VOC coatings, slashing environmental impact by 30%. Lab-to-industry examples: lignin-modified C4H4 polymers for biodegradables, or thioacetal-linked chains for recyclables. Selective hydrogenation catalysts like Pd-Cu on alumina boost C4H4 to butadiene yields, improving efficiency in mixed C4 streams. As polymer markets expand, C4H4 enables 3D-printable resins and smart hydrogels, offering unique scalability and eco-benefits.
Role in Catalysis: Catalyzing Efficiency and Sustainability in Chemical Processes
In catalysis, vinyl acetylene acts as both reactant and influencer, enhancing reactions like acetylene hydrochlorination for vinyl chloride monomer (VCM). It forms as an intermediate, but advanced catalysts minimize it to boost selectivity. Gold-based catalysts, commercialized recently, replace toxic mercury, with ultralow gold levels achieving high stability. My insights: C4H4’s presence affects ethylene polymerization, forming polymers that can foul catalysts, but density functional theory (DFT) studies optimize zinc acetate systems to mitigate this.
Recent advancements: metal-free carbon catalysts with heteroatom doping or defects excel in hydrochlorination, rivaling metals with superior durability. Single-atom catalysts, like Au on nitrogen-doped carbon, hit 94.2% conversion, stabilized by pyrrole nitrogen. P-block element-regulated catalysts form ultrafine particles for better atom efficiency. In coupled reactions, C4H4 aids sustainable chains, like CO2 valorization to C2-C6 products. Porphyrin-boosted Cu catalysts with low loading enhance activation, narrowing gaps to noble metals. These innovations make C4H4 a catalyst enabler, promoting green VCM production.
Safety, Handling, and Environmental Considerations: Prioritizing Responsible Use
Vinyl acetylene’s reactivity necessitates strict safety protocols. Flammable and explosive in air (2-100% mixtures), it risks detonation without stabilizers. From my experience, store in cooled cylinders, use non-sparking tools, and ensure ventilation. PPE includes respirators and flame-retardant gear; leaks demand immediate evacuation.
Environmentally, production generates wastewater, but recycling cuts impacts. VOC emissions are controlled via scrubbers; bio-routes reduce CO2 by 40%. Regulations like REACH mandate low emissions, pushing mercury-free catalysis. Sustainable handling ensures C4H4’s benefits outweigh risks.
Future Trends and Market Outlook: Vinyl Acetylene in a Green Industrial Landscape
By 2030, vinyl acetylene ties into acetylene’s growth to 13.58 million tons, with Asia leading. Trends: AI-optimized synthesis, CO2 electrocatalysis, and advanced hydrogenation catalysts. Emerging: plasmonic catalysts for solar-driven reactions, enhancing selectivity. Market shifts emphasize sustainability, with bio-feedstocks and coupled processes for circular economies. From my vantage, C4H4 will expand into quantum materials and bio-synthesis, driven by global defossilization.
Conclusion: Vinyl Acetylene‘s Enduring Impact from Bench to Boardroom
Vinyl acetylene (C4H4) exemplifies how lab insights propel industrial advancements in polymers and catalysis. Its reactive prowess enhances material durability, catalytic efficiency, and sustainability, offering unique solutions in a evolving market. As we embrace greener paths, leveraging C4H4 responsibly will foster innovation. Based on my career, its potential is vast—urging stakeholders to invest for a resilient future.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow up our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )