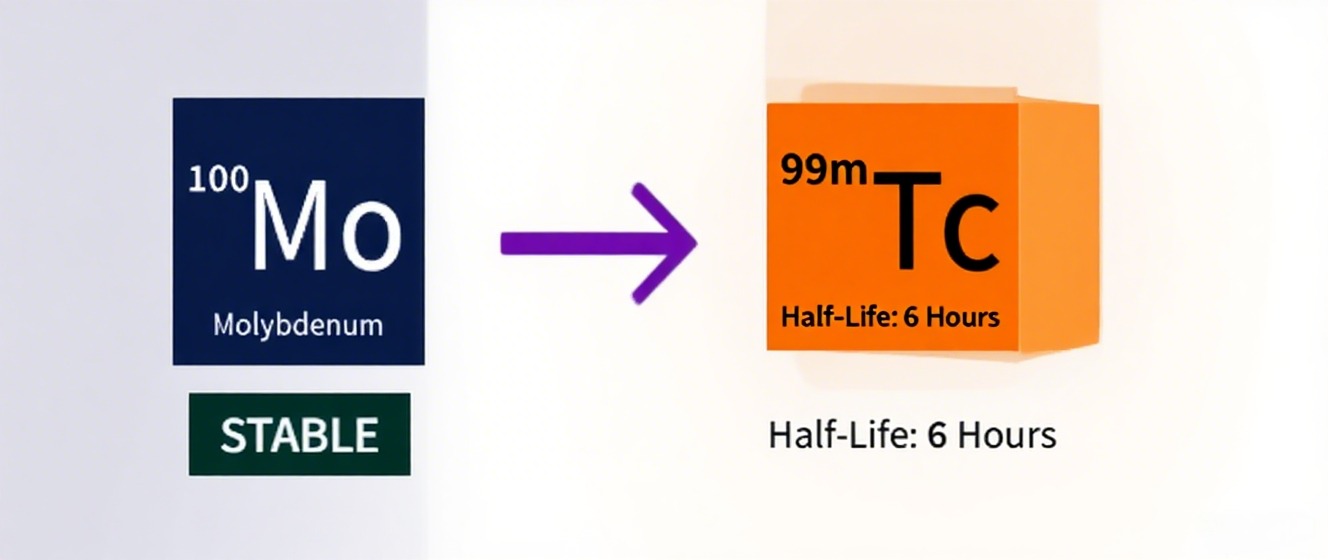
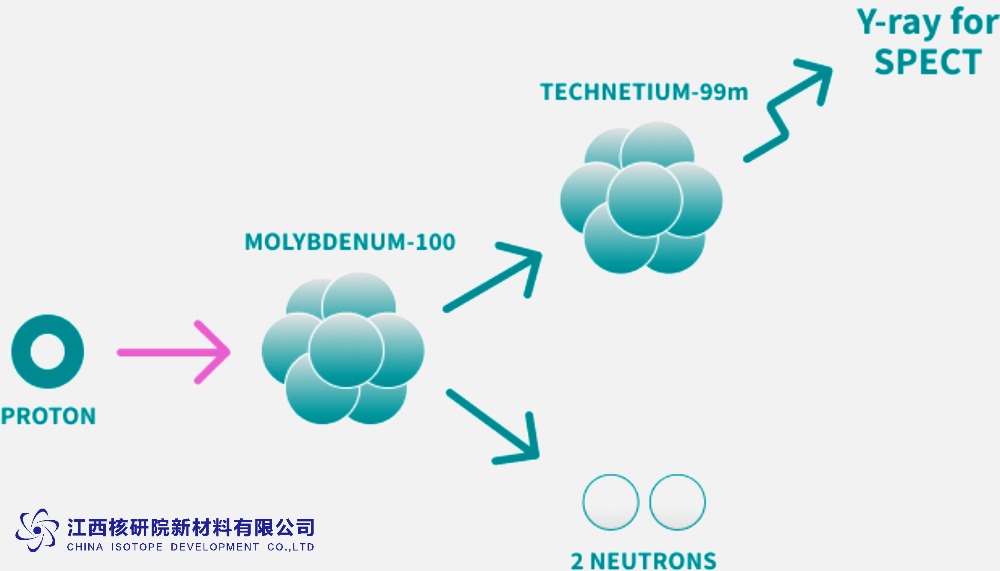
Stable Isotope Excellence: How Molybdenum-100 Drives Precision in Neutrino Studies and Beyond
BY Tao, Published August 26, 2025
In the landscape of modern nuclear science and precision metrology, stable isotopes occupy a foundational role that underpins cutting-edge research and practical applications alike. Among these, Molybdenum-100 (^100Mo) stands out as a quiet workhorse with far-reaching impact. From neutrino physics to materials science and environmental tracing, the unique properties of ^100Mo enable researchers to push the boundaries of measurement accuracy, selectivity, and interpretability. This article presents a comprehensive view of why ^100Mo matters, how its stable isotope characteristics translate into real-world advantages, and what to consider when selecting and using ^100Mo-containing materials and sources in high-precision research.
The core role of stable isotopes in modern science
Stable isotopes are non-radioactive variants of elements that differ in neutron number. They provide pristine references for tracking physical, chemical, and biological processes without the confounding effects of radioactive decay. In neutrino research, precise isotopic compositions help constrain theoretical models, calibrate detectors, and reduce systematic uncertainties that can mask subtle signals. Beyond fundamental physics, stable isotopes serve as tracers in geochemistry, environmental science, astrochemistry, and industrial processes. They enable:
- High-precision calibration of mass spectrometers and infrared spectroscopy.
- Isotopic labeling for reaction mechanism studies and kinetic analyses.
- Traceability and standardization in inter-laboratory comparisons.
- Enhanced sensitivity in materials characterization, diffusion studies, and phase analysis.
Against this backdrop, specific isotopes gain prominence due to their favorable natural abundance, ease of enrichment, chemical versatility, and compatibility with state-of-the-art instrumentation. Molybdenum-100 is one such isotope that has earned a distinctive place in the toolbox of researchers aiming for precision and reliability.
Why Molybdenum-100 is uniquely valuable
Molybdenum has seven stable isotopes in natural composition, with ^100Mo being one of the less abundant but highly useful isotopes for targeted applications. The value of ^100Mo derives from a combination of factors:
- Chemical versatility and physical stability: Molybdenum compounds exhibit a wide range of oxidation states and coordination chemistries, enabling robust incorporation into solid-state matrices, thin films, ceramics, and enrichment targets without compromising structural integrity.
- Isotopic purity and traceability: High-purity ^100Mo materials enable cleaner isotope ratio measurements, reducing cross-talk from other Mo isotopes in analytical workflows.
- Compatibility with high-precision instrumentation: The stable nature of ^100Mo allows seamless integration with mass spectrometric, spectroscopic, and detector-calibration schemes without concerns about radioactive decay backgrounds or long-lived radiological hazards.
- Enrichment feasibility: While ^100Mo is not the most abundant isotope in natural Mo, modern enrichment techniques can produce isotopically enriched targets with reasonable yield and cost profiles, enabling tailored experiments and repeatable calibrations.
In neutrino physics, the precision of isotopic composition directly influences the ability to model background signals, simulate detector responses, and interpret subtle interaction channels. For example, neutrino experiments often rely on highly controlled materials where trace isotopic impurities can mimic or obscure rare events. ^100Mo-enriched materials can serve as ultra-pure calibrants and phonon/particle interaction simulants, thereby sharpening the fidelity of the data that informs fundamental neutrino properties.
Applications in neutrino studies and beyond
Neutrino physics: calibration, background suppression, and detector response
- Detector calibration sources: Enriched ^100Mo can be incorporated into calibration targets or reference materials that interact with neutrino-induced signals in predictable ways. The stable isotope composition ensures reproducibility across calibration campaigns and laboratories.
- Background modeling: Precise knowledge of the isotopic makeup of detector components helps eliminate degeneracies in background models. ^100Mo’s stable behavior avoids complexities associated with decay chains that could otherwise complicate long-term data interpretation.
- Phonon and scintillation studies: In scintillating or phonon-detecting detectors, Mo-based compounds can influence light yield and phonon transport properties. Understanding how ^100Mo-containing matrices affect signal characteristics enhances the accuracy of energy reconstruction and event classification.
Materials science and solid-state research
- Isotopic labeling for diffusion and lattice studies: Enriched ^100Mo in solids enables high-fidelity tracking of diffusion processes, phase transitions, and defect interactions, yielding insights into material performance under extreme conditions.
- Thermal stability and corrosion resistance: Mo compounds contribute to high-temperature stability and corrosion resistance in harsh environments. Controlling isotopic composition helps distinguish intrinsic material behavior from isotopic effects.
- Thin films and ceramics: ^100Mo-enriched targets can be integrated into films and ceramic composites used in sensors and nuclear instrumentation, where isotopic purity translates into lower impurity-related noise and enhanced reproducibility.
Environmental tracing and geoscience
- Isotopic tracers for geochemical processes: Stable Mo isotopes, including ^100Mo, can be used to trace ore formation, mineralization pathways, and mobility in ecosystems, contributing to models of nutrient cycling and contaminant fate.
- Laboratory standards and inter-lab comparability: Consistent ^100Mo reference materials support cross-lab comparisons in environmental isotopic analyses, improving confidence in reported trends and flux estimates.
Product parameters and performance considerations
When selecting ^100Mo-containing materials or sources for research and instrumentation, several key parameters drive performance and reliability. The following consolidated considerations offer a practical guide for researchers and procurement specialists.
Isotopic enrichment level
- Target enrichment: Common goals include achieving a high degree of ^100Mo enrichment to maximize signal fidelity in calibration tasks or to minimize spectral overlap in mass spectrometric analyses.
- Typical enrichment ranges: Enrichment can span from near-natural abundance levels to greater than 90% ^100Mo for specialized applications. The optimal choice depends on budget, required signal-to-noise ratio, and compatibility with the hosting material.
Chemical form and material compatibility
- Solid state hosts: Mo can be incorporated into oxides, carbides, nitrides, or metallic matrices. The choice affects diffusion behavior, mechanical stability, and thermal properties.
- Solubility and compatibility: Ensure that the chosen chemical form does not introduce unwanted impurities or matrix effects that could skew isotope ratio measurements or alter detector responses.
Purity and impurity profile
- Impurity control: Trace elements (reductants, catalysts, or binding agents) can influence mass bias, ionization efficiency, or background signals. A well-characterized impurity profile is essential for high-precision experiments.
- Chemical separation requirements: In some workflows, post-processing steps such as purification or chromatographic separation may be necessary to achieve the desired isotopic purity.
Physical properties relevant to instrumentation
- Thermal and mechanical stability: Materials should withstand the thermal cycles of detector calibration or environmental testing without phase changes that affect isotopic composition or geometry.
- Surface chemistry and cleanliness: For contact-sensitive measurements (e.g., surface analysis, thin-film deposition), surface cleanliness and oxide layers can impact measurement bias and reproducibility.
Handling, safety, and regulatory considerations
- Non-radioactive handling: ^100Mo is stable and non-radioactive, which simplifies many safety and regulatory concerns compared to radiogenic isotopes. Nevertheless, standard chemical hygiene and material handling protocols apply.
- Regulatory compliance: Ensure adherence to export controls, supplier certifications, and end-use restrictions relevant to specialized isotopes and high-purity materials.
Quality assurance and traceability
- Certificate of analysis: A detailed COA documenting isotopic composition, total Mo content, and impurity levels underpins traceability and reproducibility.
- Batch-to-batch consistency: For longitudinal studies or inter-lab campaigns, selecting suppliers with stringent lot-to-lot consistency reduces systematic uncertainties.
Practical guidelines for researchers
To maximize the impact of ^100Mo in experimental workflows, consider the following best practices:
- Plan enrichment with purpose: Align enrichment levels with the specific measurement goals, balancing cost with the required isotopic purity for your calibration or experimental simulations.
- Integrate systematic uncertainty assessment early: Model how isotopic composition uncertainties propagate through your detector response or analytical method to guide material selection and calibration strategies.
- Characterize matrix effects comprehensively: Before deploying ^100Mo-containing materials, characterize any potential matrix effects on spectroscopy, mass spectrometry, or detector readouts.
- Implement rigorous cleanliness standards: Maintain clean handling and storage conditions to prevent contamination that could compromise isotope measurements, particularly in ultra-trace analyses.
- Coordinate with manufacturers on traceability: Work with reputable suppliers to obtain full traceability documentation, including production history and isotopic performance data.
Case studies: demonstrated impact of ^100Mo in precision science
- Case studies from neutrino-dedicated and materials research laboratories illustrate how ^100Mo-enriched matrices have enabled clearer calibration curves, reduced systematic uncertainties, and improved model fidelity in complex analyses. While the specifics vary by experimental design, the overarching theme is consistent: stable isotopic control translates to sharper, more interpretable data. These practical outcomes manifest as tighter confidence intervals, more robust background subtraction, and enhanced ability to discriminate between competing physical models.
Future directions and opportunities
Looking ahead, several avenues promise to broaden the utility of ^100Mo in precision science:
- Advanced enrichment technologies: Developments in catalytic, electromagnetic, or centrifuge-based enrichment methods may reduce costs and increase accessibility of high-purity ^100Mo targets.
- Hybrid materials and metamaterials: Incorporating ^100Mo into engineered matrices could tailor transport properties, optical responses, or thermal conductivities to suit next-generation detectors and sensors.
- Cross-disciplinary isotopic standards: Integrated standards that combine ^100Mo with other stable isotopes could streamline multi-parameter calibration in complex measurement systems.
Summary: maximizing the value of Molybdenum-100
Molybdenum-100 stands as a pivotal stable isotope whose thoughtful application can elevate the precision and reliability of neutrino studies, materials science, and environmental research. Its chemical versatility, favorable enrichment potential, and stable, non-radioactive nature make it a practical choice for laboratories seeking to minimize systematic uncertainties and maximize reproducibility. By carefully selecting enrichment levels, chemical forms, and purity profiles, researchers can harness the full potential of ^100Mo to deliver clearer insights, more robust models, and sharper experimental outcomes.
Product-focused note: technical parameters and usage considerations
- Isotopic enrichment level: Targeted enrichment up to >90% ^100Mo for high-precision calibration targets; lower enrichment can suffice for general reference standards depending on measurement sensitivity.
- Chemical forms: Oxides (e.g., MoO2, MoO3), nitrides, carbides, or metallic Mo powders/foils, chosen based on integration with the host matrix and specific instrument compatibility.
- Purity benchmarks: Impurity levels in trace to sub-ppm ranges for critical analyses; full impurity profiles should accompany procurement to anticipate potential mass bias or background signals.
- Physical packaging: Stable, hermetically sealed packages to prevent contamination and preserve isotopic integrity during storage and transport.
- Handling guidelines: Standard chemical hygiene; avoid moisture-sensitive routes when corrosion risk could alter surface chemistry; use inert atmospheres if reactivity is a concern.
- Quality documentation: Certificate of analysis, isotope ratio measurements, and impurity spec sheets; batch traceability is essential for long-term experiments.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow up our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )