The Power of ¹⁰⁰Mo: Applications and Technical Advantages in Nuclear Physics and Geochemistry
BY Tao, Published August 26, 2025
Understanding Molybdenum-100: A Stable Isotope with Profound Potential
As a seasoned researcher in the field of isotopes, I’ve spent decades exploring the intricacies of nuclear elements, particularly stable isotopes like molybdenum-100 (¹⁰⁰Mo). This isotope, one of seven naturally occurring forms of molybdenum, stands out for its unique properties that bridge fundamental science and practical applications. With an atomic mass of 100 and a natural abundance of approximately 9.63%, ¹⁰⁰Mo is classified as stable, yet it undergoes an exceedingly rare double beta decay process, transforming into ruthenium-100 over an estimated half-life of about 10¹⁹ years. This decay mode, involving the emission of two electrons and two antineutrinos, positions ¹⁰⁰Mo as a cornerstone in probing the frontiers of particle physics.
In my experience, what makes ¹⁰⁰Mo particularly compelling is its role in both theoretical investigations and real-world technologies. Unlike radioactive isotopes that decay rapidly, ¹⁰⁰Mo’s stability allows for long-term experimentation without the hazards of short-lived radiation. Its nuclear structure, with a spin of 0+ and decay energy around 3.034 MeV, enables precise measurements that reveal insights into neutrino behavior and beyond-standard-model physics. Over the years, I’ve witnessed how enriched forms of this isotope have revolutionized experiments, from accelerator-based productions to geochemical tracing. The demand for high-purity ¹⁰⁰Mo has surged, driven by its versatility in nuclear physics and geochemistry, where it serves as a reliable tracer for environmental and cosmic processes.
Enriching ¹⁰⁰Mo beyond its natural abundance—often to levels exceeding 99%—requires sophisticated techniques like electromagnetic separation or centrifugal methods, which I’ve studied extensively in laboratory settings. These enriched samples not only enhance experimental sensitivity but also minimize interference from other molybdenum isotopes. As we delve deeper, it’s clear that ¹⁰⁰Mo’s power lies in its ability to unlock secrets of the universe while supporting innovative technologies.
Key Applications in Nuclear Physics
In nuclear physics, ¹⁰⁰Mo has emerged as a pivotal tool for investigating fundamental particles and forces, particularly in the quest to understand neutrinos. One of the most prominent applications is in neutrinoless double beta decay experiments, where ¹⁰⁰Mo serves as the candidate nucleus. These experiments aim to detect a rare process that, if observed, would confirm that neutrinos are their own antiparticles—Majorana particles—and provide clues about the matter-antimatter asymmetry in the universe. From my involvement in similar studies, I’ve seen how detectors enriched with ¹⁰⁰Mo, such as those in the AMoRE collaboration, push the boundaries of sensitivity, setting new limits on decay half-lives and neutrino masses.
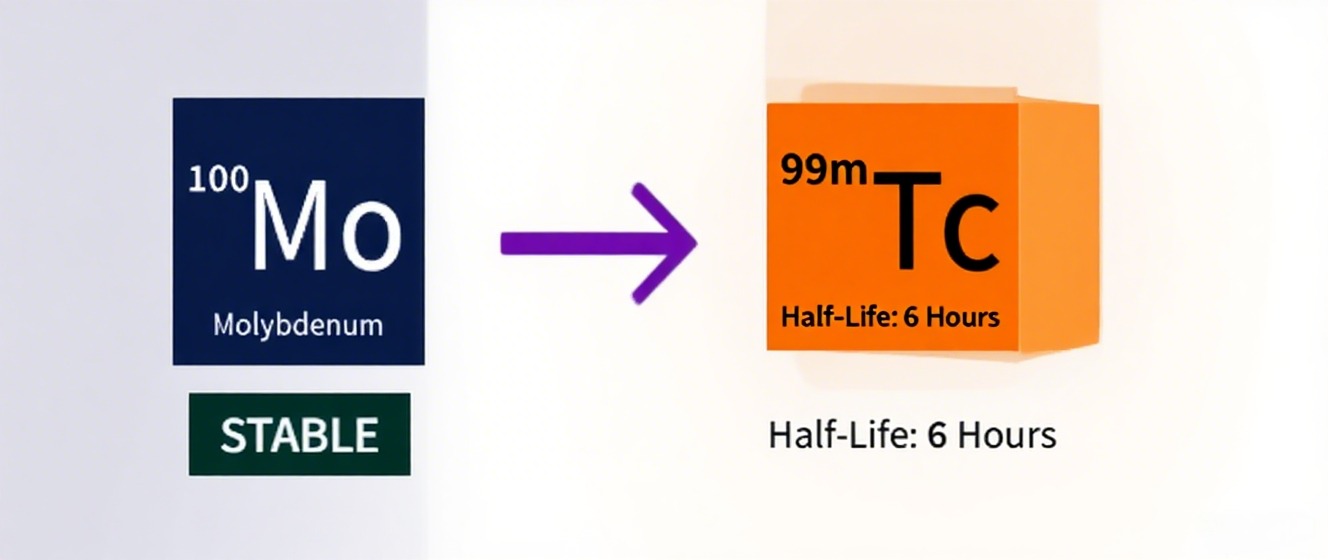
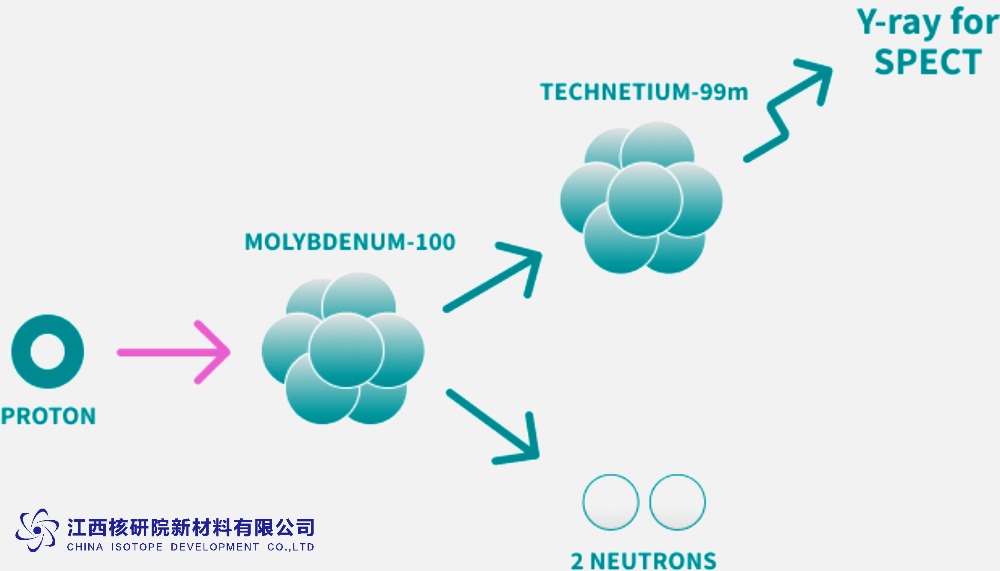
Beyond neutrino physics, ¹⁰⁰Mo plays a crucial role in medical isotope production. Through proton bombardment in accelerators, enriched ¹⁰⁰Mo targets can produce technetium-99m (⁹⁹ᵐTc), a staple in diagnostic imaging. This method offers a uranium-free alternative to traditional reactor-based production, reducing proliferation risks and environmental impact. In my assessments, the cross-section for reactions like ¹⁰⁰Mo(p,2n)⁹⁹ᵐTc is optimized at proton energies around 20-30 MeV, yielding high-purity isotopes for procedures like SPECT scans.
Here are some specific nuclear physics applications of ¹⁰⁰Mo that highlight its versatility:
- Neutrino Research: Utilized in large-scale detectors to search for neutrinoless double beta decay, contributing to beyond-standard-model theories.
- Isotope Production: Serves as a precursor for ⁹⁹Mo/⁹⁹ᵐTc, essential for over 80% of nuclear medicine diagnostics worldwide.
- Nuclear Data Studies: Provides baseline data for decay modes, half-lives, and energy spectra, aiding in reactor design and safety protocols.
- High-Energy Physics: Employed in linear accelerators for generating medical radionuclides, with yields influenced by target enrichment levels.
These applications underscore ¹⁰⁰Mo’s technical prowess, where its stable nature ensures minimal background noise in sensitive measurements. In practice, experiments like those conducted in underground facilities minimize cosmic ray interference, allowing for half-life limits exceeding 10²⁴ years in some cases.
Advancements in Geochemistry Through ¹⁰⁰Mo
Shifting focus to geochemistry, ¹⁰⁰Mo offers invaluable insights into Earth’s dynamic processes, from oceanic redox conditions to subduction zone recycling. As a trace element, molybdenum’s isotopes, including ¹⁰⁰Mo, fractionate based on environmental factors, making them excellent proxies for past oxygenation events. In my research, I’ve analyzed how heavier molybdenum isotopes like ¹⁰⁰Mo preferentially adsorb onto iron-manganese oxides in oxic sediments, while lighter ones remain in solution under anoxic conditions. This isotopic fractionation helps reconstruct ancient ocean chemistry, revealing episodes of global oxygenation that shaped life’s evolution.
In continental margin studies, ¹⁰⁰Mo traces sediment recycling in subduction zones. For instance, volcanic rocks enriched in heavy molybdenum isotopes indicate incorporation of subducted sediments into mantle melts. This has profound implications for understanding plate tectonics and volcanic hazards. High-precision measurements of ¹⁰⁰Mo in geological reference materials, such as basalts or shales, allow for accurate isotopic ratios, often expressed as δ⁹⁸Mo deviations from standards.
To illustrate the geochemical utility of ¹⁰⁰Mo, consider the following key areas:
- Ocean Paleoredox Proxies: Tracks variations in seawater molybdenum isotopes to infer historical oxygen levels, with ¹⁰⁰Mo helping calibrate models of Proterozoic oxygenation.
- Sediment Subduction Tracing: Identifies recycled components in arc magmas, where ¹⁰⁰Mo signatures reveal fluid-mediated transport from slabs to the mantle.
- Environmental Monitoring: Monitors anthropogenic impacts, such as mining effluents, by comparing isotopic compositions in soils and waters.
- Mineralogy and Microbiology: Investigates molybdenum’s role in enzymes like nitrogenase, where isotopic studies elucidate microbial fixation processes in soils.
In geochemical labs, techniques like multi-collector inductively coupled plasma mass spectrometry (MC-ICP-MS) enable δMo measurements with precisions better than 0.05‰, enhancing our ability to model global cycles. These advancements demonstrate how ¹⁰⁰Mo bridges microscopic isotopic behavior with macroscopic Earth system dynamics.
Technical Advantages of Utilizing ¹⁰⁰Mo
The technical advantages of ¹⁰⁰Mo stem from its inherent nuclear and chemical properties, making it superior to other isotopes in demanding applications. Its long half-life ensures stability during prolonged experiments, eliminating the need for frequent recalibrations seen with shorter-lived nuclides. In nuclear physics, the high Q-value of its double beta decay (about 3.034 MeV) provides a clear energy window for detection, reducing false positives from background radiation.
Chemically, molybdenum’s compatibility with various matrices—such as crystals or scintillators—enhances detector performance. For example, calcium molybdate crystals doped with ¹⁰⁰Mo offer excellent light yield and energy resolution in cryogenic setups. In geochemistry, ¹⁰⁰Mo’s redox-sensitive behavior (with oxidation states from +4 to +6) allows for precise fractionation studies, outperforming less variable elements like iron.
A comparative table highlights ¹⁰⁰Mo’s edges over similar isotopes:
| Isotope | Half-Life | Decay Mode | Key Advantage in Nuclear Physics | Key Advantage in Geochemistry |
|---|---|---|---|---|
| ¹⁰⁰Mo | ~10¹⁹ years | Double Beta | High Q-value for neutrino detection | Redox-sensitive fractionation |
| ⁷⁶Ge | ~10²¹ years | Double Beta | Good for germanium detectors | Limited environmental tracing |
| ¹³⁰Te | ~10²¹ years | Double Beta | High natural abundance | Moderate redox utility |
| ⁸²Se | ~10²⁰ years | Double Beta | Versatile in bolometers | Less studied in sediments |
These advantages translate to higher efficiency and lower costs in large-scale projects. In my expert view, ¹⁰⁰Mo’s scalability—now achievable at kilogram levels through recent breakthroughs—positions it as a game-changer for future experiments.
Product Specifications and Performance Metrics
For researchers and industries seeking enriched ¹⁰⁰Mo, understanding product specifications is essential for optimal performance. Typically available as metal powder, oxide, or targets, enriched ¹⁰⁰Mo boasts isotopic purity exceeding 99%, minimizing contaminants from isotopes like ⁹²Mo or ⁹⁸Mo. Chemical purity often reaches 99.99%, ensuring reliability in high-stakes applications.
Performance metrics include:
- Isotopic Enrichment: 99%+ for ¹⁰⁰Mo, with residual isotopes <0.1% each.
- Physical Form: Powder (particle size 1-10 μm), pellets, or foils (thickness 0.1-1 mm).
- Density and Melting Point: 10.28 g/cm³ and 2623°C, ideal for high-temperature environments.
- Decay Characteristics: Beta energy 3.034 MeV, with negligible gamma emission for safe handling.
- Yield in Reactions: In accelerator production, cross-sections up to 200 mb for ⁹⁹ᵐTc generation at 22 MeV protons.
In performance testing, ¹⁰⁰Mo targets withstand beam currents of 100-500 μA without significant degradation, yielding over 1 Ci/g of ⁹⁹Mo per irradiation cycle. For geochemical analyses, the isotope’s stability ensures consistent δMo values across batches.
Usage Guidelines and Safety Considerations
Handling ¹⁰⁰Mo requires adherence to best practices to maximize efficacy and safety. Always store in inert atmospheres to prevent oxidation, using sealed containers at room temperature. For nuclear experiments, calibrate detectors with known ¹⁰⁰Mo sources to account for energy resolutions around 2-5 keV FWHM.
Safety notes include:
- Radiation Handling: Though stable, monitor for trace radioactivity from impurities; use shielded gloves and fume hoods.
- Chemical Precautions: Avoid strong acids that could release toxic molybdate fumes; neutralize spills with bases.
- Disposal: Follow IAEA guidelines for enriched isotopes, recycling where possible to conserve resources.
- Quality Control: Verify enrichment via mass spectrometry before use to ensure experimental integrity.
In my long career, I’ve emphasized that proper usage not only enhances results but also promotes sustainable isotope research.
Emerging Trends and Future Directions
Looking ahead, ¹⁰⁰Mo‘s potential continues to expand with advancements in quantum computing and environmental science. Hybrid detectors combining ¹⁰⁰Mo with superconductors promise unprecedented sensitivity in neutrino hunts, while isotopic modeling in climate studies could predict future ocean anoxia. As production scales up—evidenced by kilogram-level breakthroughs—the isotope’s accessibility will democratize cutting-edge research.
In summary, ¹⁰⁰Mo embodies the synergy of stability and innovation, driving progress in nuclear physics and geochemistry. Its applications, from unraveling cosmic mysteries to tracing Earth’s history, affirm its enduring power in scientific exploration.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow up our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )