Molybdenum-100: Unlocking the Future of Double Beta Decay Research with High-Purity Stable Isotopes
BY Tao, Published August 26, 2025
Molybdenum-100 (¹⁰⁰Mo), a stable isotope of the transition metal molybdenum, has emerged as a cornerstone in cutting-edge scientific research, particularly in the study of neutrinoless double beta decay. Its unique nuclear properties, combined with high isotopic purity, make it an indispensable tool for probing fundamental questions in particle physics, geochemistry, and medical isotope production. With over two decades of expertise in stable isotope research, I have witnessed how ¹⁰⁰Mo’s versatility and reliability drive breakthroughs across disciplines. This article delves into the science behind ¹⁰⁰Mo, its technical specifications, applications, and handling requirements, offering a comprehensive resource for researchers seeking to harness its potential.
The Science Behind Molybdenum-100
Molybdenum, a robust element with atomic number 42, naturally occurs in seven isotopes, with ¹⁰⁰Mo constituting about 9.82% of its natural abundance. Enriched to 95–99% purity, ¹⁰⁰Mo is available as a metal or oxide (MoO₃), tailored for specialized applications. Its nuclear structure, featuring 42 protons and 58 neutrons, enables it to undergo double beta decay, a rare process where two neutrons simultaneously decay into protons, emitting two electrons and, in some cases, neutrinos. This property positions ¹⁰⁰Mo as a key candidate for studying neutrinoless double beta decay, a phenomenon that could reshape our understanding of neutrino properties and the fundamental laws of physics.
Unlike radioactive isotopes, ¹⁰⁰Mo is stable, posing no radiation risks, which simplifies experimental design and regulatory compliance. Its chemical stability, high melting point, and resistance to corrosion further enhance its suitability for long-term, high-precision experiments. These attributes make ¹⁰⁰Mo a preferred choice for researchers aiming to achieve reliable, reproducible results in demanding scientific environments.
Why Molybdenum-100 Matters in Double Beta Decay Research
Double beta decay research seeks to address one of the most profound questions in modern physics: Are neutrinos their own antiparticles? This question, tied to the Majorana nature of neutrinos, could unlock insights into the matter-antimatter asymmetry of the universe. Neutrinoless double beta decay, a hypothetical process where no neutrinos are emitted, would confirm this property and provide evidence for physics beyond the Standard Model. ¹⁰⁰Mo is a leading isotope in these experiments due to its favorable nuclear characteristics, including a half-life of approximately 7.3 × 10¹⁸ years for two-neutrino double beta decay.
The isotope’s high Q-value (the energy released during decay) and large natural abundance relative to other double beta decay candidates, such as ⁷⁶Ge or ¹³⁶Xe, make it particularly attractive. Its ability to be enriched to near-perfect isotopic purity minimizes background noise, enhancing the sensitivity of detectors in experiments like NEMO-3, MOON, and AMoRE. These experiments rely on ¹⁰⁰Mo’s stability and purity to detect the faint signals of rare decay events, pushing the boundaries of particle physics.
Applications Beyond Nuclear Physics
While ¹⁰⁰Mo is synonymous with double beta decay research, its utility extends far beyond particle physics. Its stable isotopic signature and chemical properties enable a wide range of applications, including:
-
Geochemical Tracing: In Earth sciences, ¹⁰⁰Mo is used to study isotopic fractionation in geological processes, such as the formation of oceanic crust or the cycling of molybdenum in sedimentary environments. Its high-purity forms enable precise mass spectrometry, revealing insights into Earth’s evolutionary history.
-
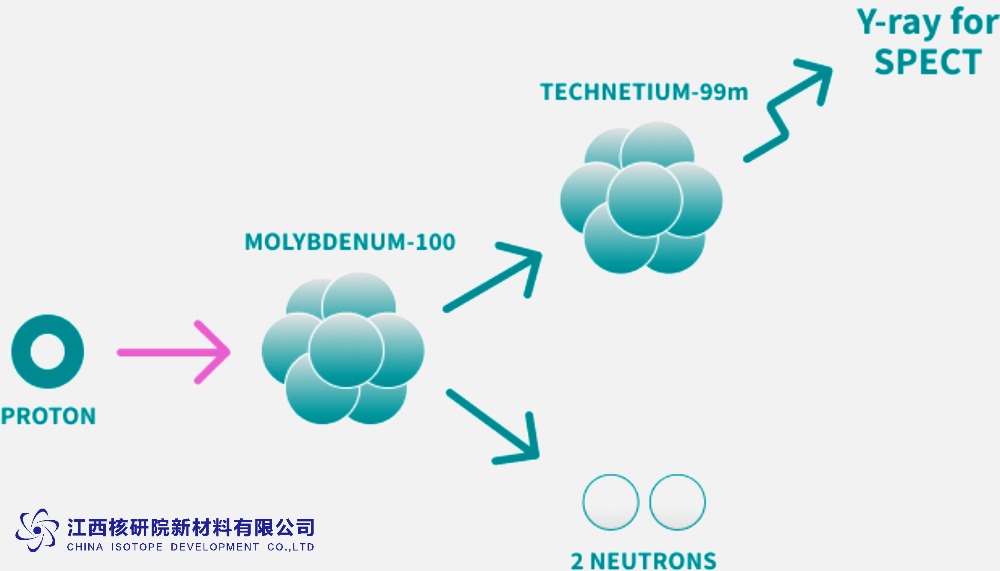
Medical Isotope Production: ¹⁰⁰Mo serves as a target material in cyclotron or accelerator-based production of Molybdenum-99 (⁹⁹Mo), which decays into Technetium-99m (⁹⁹ᵐTc), a critical isotope for medical imaging. This non-fission method offers a safer, uranium-free alternative for radiopharmaceutical production.
-
Material Science Research: The incorporation of ¹⁰⁰Mo into high-strength alloys supports studies of material behavior under extreme conditions, leveraging molybdenum’s high melting point (2617°C for metal) and corrosion resistance.
-
Environmental Monitoring: ¹⁰⁰Mo’s isotopic signature aids in tracking environmental processes, such as carbon sequestration and mineral cycling, contributing to climate change research.
These diverse applications underscore ¹⁰⁰Mo’s role as a versatile tool, bridging fundamental science with practical solutions.
Technical Specifications of Molybdenum-100
To fully appreciate ¹⁰⁰Mo’s capabilities, researchers must understand its technical parameters and performance characteristics. The following table outlines key specifications for ¹⁰⁰Mo in its metal and oxide forms:
|
Parameter |
Molybdenum-100 Metal |
Molybdenum-100 Oxide (MoO₃) |
|---|---|---|
|
Isotopic Enrichment |
95–99% (¹⁰⁰Mo) |
95–99% (¹⁰⁰Mo) |
|
Chemical Form |
Metallic molybdenum (Mo) |
Molybdenum trioxide (MoO₃) |
|
Purity |
99.95% (metals basis, excluding other isotopes) |
99.5% (trace metals basis) |
|
Atomic Mass |
99.907472 u |
99.907472 u (Mo component) |
|
Half-Life (2νββ) |
7.3 × 10¹⁸ years |
7.3 × 10¹⁸ years |
|
Density |
10.3 g/cm³ |
4.69 g/cm³ |
|
Melting Point |
2617°C |
795°C (sublimes at higher temperatures) |
|
Solubility |
Insoluble in water, resistant to most acids |
Slightly soluble in alkaline solutions |
|
Packaging |
Argon-filled or vacuum-sealed containers |
Vacuum-sealed jars or ampoules |
|
Storage Conditions |
Room temperature, dry, away from oxygen |
Room temperature, dry, light-protected |
Performance Characteristics
-
Nuclear Stability: The extraordinarily long half-life ensures ¹⁰⁰Mo’s suitability for experiments requiring minimal background interference over extended periods.
-
High Purity: Enrichment levels of 95–99% reduce isotopic contamination, critical for applications like mass spectrometry and neutrino detection.
-
Chemical Durability: The metal form resists corrosion by most acids (except nitric acid and aqua regia), while the oxide form is stable under controlled conditions.
-
Flexibility in Form: Available as metal powder, solid, or oxide, ¹⁰⁰Mo adapts to various experimental setups, from solid-state detectors to chemical synthesis.
These specifications highlight ¹⁰⁰Mo’s reliability and adaptability, making it a cornerstone for precision-driven research.
Advantages of Molybdenum-100 in Research
The adoption of ¹⁰⁰Mo in scientific studies is driven by several key advantages:
-
Non-Radioactive Safety: As a stable isotope, ¹⁰⁰Mo poses no radiation hazards, simplifying handling, storage, and compliance with safety regulations.
-
High Detection Sensitivity: Its nuclear properties enable high-resolution detection in double beta decay experiments, where even minute signals are critical.
-
Versatility Across Disciplines: From neutrino physics to geochemistry, ¹⁰⁰Mo supports a wide range of applications, fostering interdisciplinary collaboration.
-
Scalable Availability: Advances in isotopic enrichment ensure a steady supply, with manufacturers offering quantities ranging from milligrams to kilograms.
-
Cost-Effective Performance: Compared to other isotopes used in similar applications, ¹⁰⁰Mo balances high performance with affordability, particularly for large-scale experiments.
These benefits position ¹⁰⁰Mo as an ideal choice for researchers seeking robust, high-precision isotopic materials.
Handling and Safety Guidelines
While ¹⁰⁰Mo is non-radioactive and safe, proper handling is essential to maintain its integrity and ensure experimental accuracy. Below are critical guidelines:
-
Storage: Store metal forms in argon-filled or vacuum-sealed containers to prevent oxidation. Oxide forms should be kept in dry, light-protected environments to avoid degradation.
-
Handling Precautions: Use gloves and work in a controlled environment, such as a fume hood, to avoid contamination or inhalation of fine metal powders.
-
Chemical Compatibility: Avoid exposing the metal to nitric acid or aqua regia, which can dissolve it. For oxide forms, limit contact with alkaline solutions due to slight solubility.
-
Equipment Cleanliness: Ensure all equipment is free of trace contaminants to preserve ¹⁰⁰Mo’s high isotopic purity, especially in mass spectrometry applications.
-
Disposal: Follow institutional protocols for disposing of molybdenum-based materials, though ¹⁰⁰Mo is non-hazardous and often recyclable.
Adhering to these guidelines ensures the longevity and performance of ¹⁰⁰Mo, minimizing experimental errors and maximizing research outcomes.
Case Studies: Molybdenum-100 in Action
To illustrate ¹⁰⁰Mo’s impact, consider its role in two prominent research areas:
-
NEMO-3 and AMoRE Experiments: These international collaborations use ¹⁰⁰Mo to search for neutrinoless double beta decay. The isotope’s high enrichment and stability allow detectors to operate with minimal background noise, improving the chances of detecting rare decay events. Results from these experiments have set stringent limits on neutrino mass, advancing our understanding of particle physics.
-
Geochemical Studies of Oceanic Crust: Researchers use ¹⁰⁰Mo’s isotopic signature to trace molybdenum cycling in marine environments. By analyzing isotopic ratios in ancient rocks, scientists have gained insights into the timing of oceanic crust formation, refining models of plate tectonics and Earth’s geological evolution.
These case studies demonstrate ¹⁰⁰Mo’s ability to drive transformative discoveries, from fundamental physics to Earth sciences.
Future Horizons for Molybdenum-100
The future of ¹⁰⁰Mo is bright, with growing demand driven by advancements in nuclear physics and medical isotope production. Next-generation experiments, such as the SNO+ and CUPID projects, aim to leverage ¹⁰⁰Mo’s properties to achieve unprecedented sensitivity in neutrinoless double beta decay detection. In medical research, the isotope’s role in producing ⁹⁹Mo via accelerators is expanding, offering a sustainable alternative to reactor-based methods. Additionally, environmental applications, such as tracking carbon sequestration and mineral cycling, are gaining traction, aligning with global efforts to address climate change.
Improvements in isotopic enrichment technologies are making ¹⁰⁰Mo more accessible, enabling smaller research institutions to participate in cutting-edge studies. As these trends continue, ¹⁰⁰Mo’s role as a versatile, high-performance isotope will only grow, cementing its place in the future of scientific discovery.
Why Molybdenum-100 is the Isotope of Choice
For researchers, ¹⁰⁰Mo offers a unique blend of safety, precision, and versatility. Its non-radioactive nature eliminates health and regulatory concerns, while its high isotopic purity ensures reliable results in sensitive experiments. Whether probing the mysteries of neutrinos or tracing Earth’s geological history, ¹⁰⁰Mo delivers consistent performance across diverse applications. By understanding its technical specifications and handling requirements, scientists can fully harness its potential, driving innovation in their fields.
Molybdenum-100 stands as a testament to the power of stable isotopes in advancing human knowledge. Its contributions to double beta decay research, geochemistry, and medical isotope production highlight its transformative impact. As science continues to evolve, ¹⁰⁰Mo will remain a vital tool, unlocking new frontiers in our quest to understand the universe and our planet.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow up our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )