Comprehensive Guide to Molybdenum-100: Parameters, Performance, and Applications in Advanced Science
Molybdenum-100 (¹⁰⁰Mo) stands as a pivotal stable isotope in the realm of advanced scientific research, offering unparalleled precision in nuclear physics, geochemistry, and medical isotope production. With its unique nuclear properties, including a long half-life for double beta decay studies, ¹⁰⁰Mo has become indispensable for researchers seeking to unravel the mysteries of fundamental particles and Earth’s geochemical cycles. Drawing from decades of expertise in isotope applications, this guide explores the technical specifications, performance characteristics, and transformative applications of Molybdenum-100, providing scientists with a clear understanding of its value and handling requirements.
What is Molybdenum-100?
Molybdenum, a silvery-white transition metal, exists naturally in seven isotopes, with ¹⁰⁰Mo comprising approximately 9.82% of natural molybdenum. Enriched ¹⁰⁰Mo, typically available in metal or oxide (MoO₃) form, is processed to achieve isotopic purities of 95–99%, making it ideal for specialized applications. Its nuclear structure, with 42 protons and 58 neutrons, grants it stability and unique decay characteristics, notably its role in double beta decay, a process critical to neutrino physics. Unlike radioactive isotopes, ¹⁰⁰Mo poses no significant radiation risk, making it a safe choice for long-term experimental setups.
Key Applications of Molybdenum-100
The versatility of ¹⁰⁰Mo stems from its nuclear and chemical properties, enabling its use across diverse scientific fields. Below are its primary applications:
-
Nuclear Physics and Neutrino Studies: ¹⁰⁰Mo is a prime candidate for studying neutrinoless double beta decay, a rare process that could confirm the Majorana nature of neutrinos and inform theories beyond the Standard Model of particle physics. Its long half-life of approximately 7.3 × 10¹⁸ years ensures stable experimental conditions over extended periods.
-
Geochemical Tracing: In Earth sciences, ¹⁰⁰Mo serves as a tracer for understanding isotopic fractionation in geological processes, such as oceanic crust formation and mineral deposition. Its high isotopic purity enhances the accuracy of mass spectrometry analyses.
-
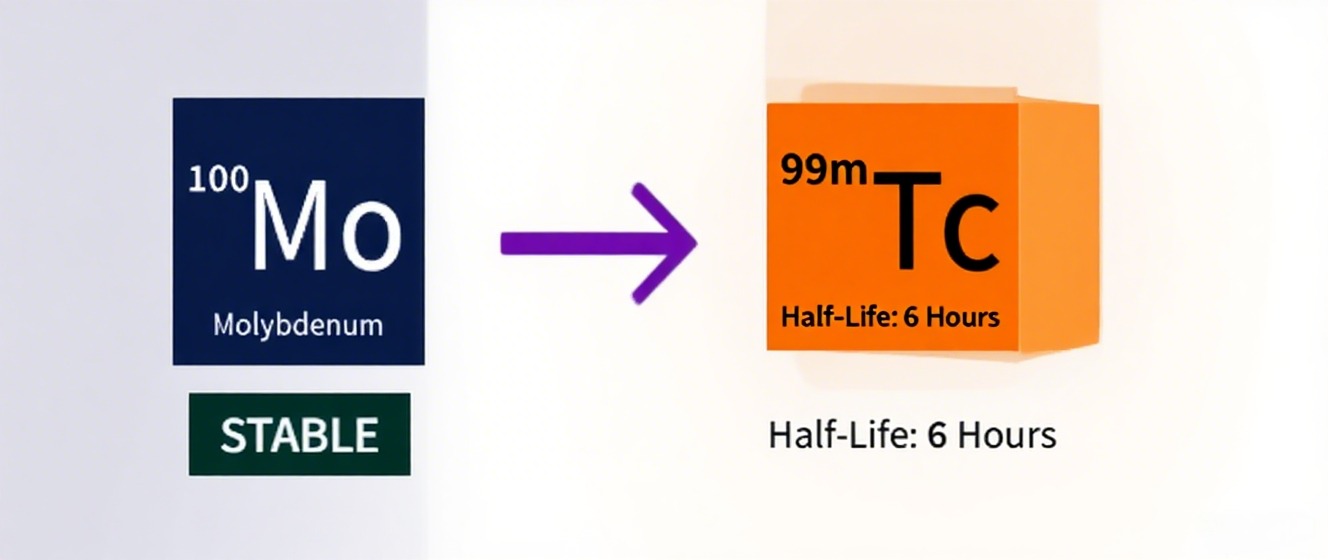
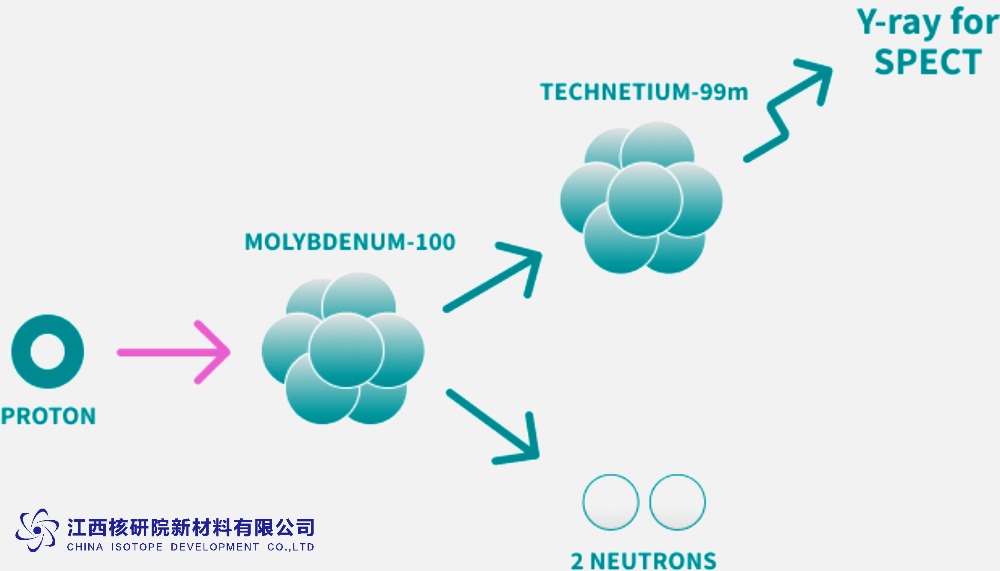
Medical Isotope Production: ¹⁰⁰Mo is used in accelerator-based methods to produce Molybdenum-99 (⁹⁹Mo), a precursor to Technetium-99m (⁹⁹ᵐTc), widely employed in medical imaging. Proton bombardment or photoneutron reactions on ¹⁰⁰Mo targets offer a non-fission alternative to traditional uranium-based methods.
-
Material Science: The isotope’s incorporation into high-strength alloys supports research into material properties under extreme conditions, leveraging molybdenum’s high melting point and corrosion resistance.
These applications highlight ¹⁰⁰Mo’s role in advancing both fundamental and applied sciences, from probing subatomic particles to enhancing medical diagnostics.
Technical Specifications and Performance
To maximize the utility of ¹⁰⁰Mo, researchers must understand its precise parameters and performance characteristics. The following table summarizes key specifications for high-purity ¹⁰⁰Mo in metal and oxide forms:
|
Parameter |
Molybdenum-100 Metal |
Molybdenum-100 Oxide (MoO₃) |
|---|---|---|
|
Isotopic Enrichment |
95–99% (¹⁰⁰Mo) |
95–99% (¹⁰⁰Mo) |
|
Chemical Form |
Metallic molybdenum (Mo) |
Molybdenum trioxide (MoO₃) |
|
Purity |
99.95% (metals basis, excluding other isotopes) |
99.5% (trace metals basis) |
|
Atomic Mass |
99.907472 u |
99.907472 u (Mo component) |
|
Half-Life |
7.3 × 10¹⁸ years |
7.3 × 10¹⁸ years |
|
Density |
10.3 g/cm³ |
4.69 g/cm³ |
|
Melting Point |
2617°C |
795°C (sublimes at higher temperatures) |
|
Solubility |
Insoluble in water, resistant to most acids |
Slightly soluble in alkaline solutions |
|
Packaging |
Argon-filled containers or vacuum-sealed |
Vacuum-sealed jars or ampoules |
|
Storage Conditions |
Room temperature, away from moisture and oxygen |
Room temperature, dry, light-protected |
Performance Highlights
-
Nuclear Stability: With a half-life of 7.3 × 10¹⁸ years, ¹⁰⁰Mo is ideal for long-term experiments, particularly in detecting rare decay events with high sensitivity.
-
Chemical Inertness: The metal form resists corrosion by most acids (except nitric acid and aqua regia), ensuring durability in harsh experimental environments.
-
High Purity: Enrichment levels up to 99% minimize interference from other molybdenum isotopes, critical for precise isotopic ratio measurements.
-
Versatile Forms: Available as metal powder, solid, or oxide, ¹⁰⁰Mo accommodates diverse experimental setups, from solid-state detectors to chemical synthesis.
These specifications underscore ¹⁰⁰Mo’s reliability as a stable isotope for demanding applications, offering both flexibility and precision.
Advantages of Using Molybdenum-100
The adoption of ¹⁰⁰Mo in research is driven by several technical and practical advantages:
-
Non-Radioactive Safety: Unlike radioactive isotopes, ¹⁰⁰Mo poses no health risks, simplifying handling and storage while reducing regulatory burdens.
-
High Sensitivity in Detection: Its nuclear properties enable high-resolution detection in experiments like neutrinoless double beta decay, where even minute signals are critical.
-
Broad Applicability: From fundamental physics to applied geochemistry, ¹⁰⁰Mo’s versatility supports interdisciplinary research, enhancing collaboration across fields.
-
Scalable Production: Advances in isotopic enrichment techniques ensure consistent supply, with manufacturers offering custom quantities from milligrams to kilograms.
-
Cost-Effectiveness: Compared to other isotopes used in similar applications, ¹⁰⁰Mo offers a balance of performance and affordability, especially for large-scale experiments.
These benefits position ¹⁰⁰Mo as a preferred choice for researchers seeking reliable, high-performance isotopic materials.
Usage Precautions and Handling Guidelines
While ¹⁰⁰Mo is stable and non-radioactive, proper handling is essential to maintain its integrity and ensure experimental accuracy. Below are key precautions:
-
Storage: Store in airtight, argon-filled, or vacuum-sealed containers to prevent oxidation, especially for metal forms. Oxide forms should be kept away from moisture and light to avoid degradation.
-
Handling: Use gloves and work in a controlled environment to avoid contamination. Metal powders are fine and may pose inhalation risks if not handled in a fume hood.
-
Chemical Compatibility: Avoid exposure to nitric acid or aqua regia, which can dissolve the metal. For oxide forms, alkaline solutions should be used cautiously due to slight solubility.
-
Purity Maintenance: Ensure equipment is free of trace contaminants to preserve the high isotopic enrichment of ¹⁰⁰Mo, particularly in mass spectrometry applications.
-
Disposal: Follow institutional guidelines for disposing of molybdenum-based materials, though ¹⁰⁰Mo is non-hazardous and typically recyclable.
Adhering to these guidelines ensures the longevity and performance of ¹⁰⁰Mo in research settings, minimizing experimental errors.
Real-World Impact: Case Studies
To illustrate ¹⁰⁰Mo’s transformative potential, consider its role in two key areas:
-
Neutrinoless Double Beta Decay Experiments: Projects like the NEMO-3 and CUORE experiments utilize ¹⁰⁰Mo to search for neutrinoless double beta decay, a process that could redefine our understanding of neutrino mass and particle physics. The isotope’s high enrichment and stability enable detectors to operate with minimal background noise, enhancing the likelihood of detecting rare events.
-
Geochemical Isotope Tracing: In studies of Earth’s mantle evolution, ¹⁰⁰Mo’s isotopic signature helps researchers distinguish between primordial and recycled crustal materials. Its use in high-precision mass spectrometry has clarified the timing of oceanic crust formation, contributing to models of plate tectonics.
These examples demonstrate how ¹⁰⁰Mo bridges fundamental research with practical applications, driving discoveries that resonate across scientific disciplines.
Future Prospects for Molybdenum-100
The demand for ¹⁰⁰Mo is poised to grow as advancements in nuclear physics and medical isotope production accelerate. Emerging technologies, such as next-generation particle detectors and cyclotron-based ⁹⁹Mo production, rely on the isotope’s unique properties. Additionally, its role in environmental science is expanding, with applications in tracking carbon sequestration and mineral cycling gaining traction. As isotopic enrichment techniques improve, ¹⁰⁰Mo’s accessibility and affordability will further democratize its use, empowering smaller research institutions to participate in cutting-edge studies.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )