Phosphorus Trifluoride: The Essential Gas for Next-Generation Material Processing
BY TAO, Published August 21, 2025
Phosphorus trifluoride (PF3) stands at the crossroads of modern materials processing, bridging fundamental chemistry with the demands of advanced manufacturing. In the realm of specialty gases, PF3 is recognized for its unique reactivity, reliable performance under demanding conditions, and its role in enabling precise, highly controlled processing steps. This article provides a comprehensive, expert-level view of PF3, including its properties, applications, handling considerations, and practical guidance for users seeking to leverage it in next-generation material processing.
1. Understanding PF3: Key properties and behavior
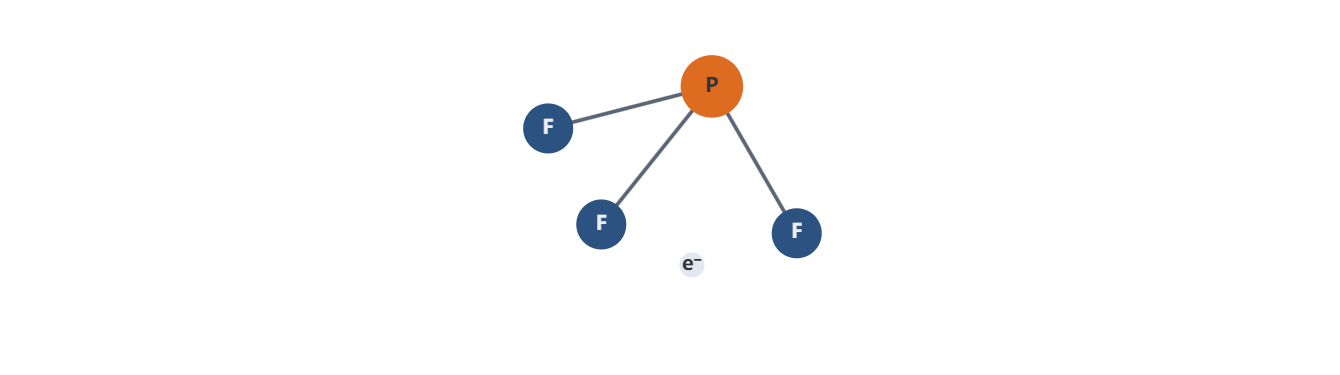
- Molecular identity and basic properties: PF3 is a trigonal pyramidal molecule consisting of a phosphorus center bonded to three fluorine atoms. It is a gas at standard conditions with a boiling point significantly lower than ambient temperatures, enabling easy handling in gas delivery systems. PF3 is highly reactive, particularly with oxygen and moisture, and it participates in a range of chemical transformations common in semiconductor and materials processing.
- Reactivity profile: PF3 exhibits strong P–F bond character and can act as a fluorinating agent in carefully controlled environments. Its reactivity is highly sensitive to temperature, pressure, and the presence of reducing agents, making system design and gas purity critical considerations for production environments.
- Physical state and transport considerations: As a gaseous species, PF3 is typically delivered via high-purity cylinder gas sources or on-site generation, with rigorous specifications for impurity levels, residual moisture, and hydrolytic stability. Its transport and storage require materials compatibility (often stainless steels or fluoropolymer-lined systems) to minimize corrosion and adsorption losses.
2. Why PF3 is essential for next-generation material processing
- Precision fluorination and surface modification: PF3 provides a controlled fluorinating capability that supports selective surface functionalization, passivation layers, and diffusion barrier formation. In advanced microfabrication and 3D materials processing, precise fluorine delivery helps tailor surface energies, adhesion properties, and etch selectivity.
- Compatibility with high-purity workflows: In environments where process purity dictates device yield and material performance, PF3’s behavior as a high-purity gas with well-defined impurity profiles makes it suitable for integration into cleanroom-grade gas delivery systems.
- Complementarity with other specialty gases: PF3 is often employed in conjunction with other fluorinated and halogenated gases to achieve complex process chemistries. Its predictable reactivity enables designers to orchestrate multi-gas sequences that achieve selective layer formation, thinning, or etching with minimal collateral damage to underlying substrates.
3. Applications across industries
- Semiconductor manufacturing: PF3 plays a role in surface activation steps, selective fluorination of protective coatings, and the formation of fluorinated interfaces that improve device resilience. Its use is typically targeted toward process steps where controlled fluorination yields enhanced etch selectivity, reduced roughness, or improved adhesion of subsequent layers.
- Advanced materials and coatings: In protective coatings and functional films, PF3 can assist in creating fluorinated terminators that reduce permeability, enhance chemical resistance, or tune optical properties. Its inclusion in process gas chemistries enables unique film compositions that are difficult to achieve with non-fluorinated precursors alone.
- Carbon-based materials and nanostructures: PF3’s fluorinating capabilities can influence the surface chemistry of carbon materials, enabling functionalization routes that improve dispersion, compatibility with polymers, or subsequent chemical modification steps essential for device integration.
4. Product parameters and performance indicators
- Purity levels: High-purity PF3 is typically supplied with stringent impurity specifications, including low moisture (< a few ppm) and minimal oxygen content, to prevent unintended side reactions that could compromise process outcomes.
- Stability and hydrolysis tolerance: PF3 reacts with moisture to form phosphorus oxyfluoride species and hydrogen fluoride, which can alter process performance and corrode delivery systems. Therefore, moisture control in the gas handling train is critical, and many systems employ in-line moisture monitoring and scrubbers.
- Cylinder and regulator compatibility: Gas cylinders for PF3 are designed to minimize adsorption and outgassing. Regulators, tubing, and valves should be selected for chemical compatibility with fluorinated compounds. Fluoropolymer liners or stainless steel components with compatible seals are commonly used to ensure integrity over the gas’s lifecycle.
- Flow and delivery control: Mass-flow controllers (MFCs) and precision regulators must be calibrated to PF3’s molar flow characteristics. Because PF3 can exhibit varying reactivity at different temperatures, process engineers typically implement tight temperature control around the delivery manifold to maintain consistent gas composition at the point of use.
- Safety and exposure limits: PF3 is a reactive gas with potential health and safety risks. Proper containment, leak detection, ventilation, and personal protective equipment are essential. Engineers must design systems with robust sealing, appropriate scrubbers, and emergency shutoff protocols.

5. Handling and safety considerations
- Hazard assessment: PF3 is reactive with moisture and oxidizing agents. It can form corrosive byproducts upon hydrolysis, so dry handling conditions are mandatory. Risk assessments should address exposure limits, inhalation hazards, and potential chemical burn risks.
- Engineering controls: Use closed-loop gas delivery with leak-tight fittings, certified compatible seals, and in-line moisture scrubbers. Ensure adequate exhaust and gas cabinet ventilation. Regular leak testing and preventive maintenance are essential to prevent environmental release and personnel exposure.
- Storage and transport: Store PF3 in designated gas cabinets or outdoor storage with secondary containment and appropriate shielding. Temperature and humidity controls reduce degradation risks and impurity formation. Use of proper labeling, inventory tracking, and traceability supports safe handling.
- Emergency response: Establish clear procedures for leaks, spills, or accidental release. Ensure availability of compatible absorbent materials, scrubbers, and emergency contact protocols. Training for personnel on PF3-specific hazards and response actions is essential for safe operation.
6. Process integration: system design and best practices
- Gas delivery architecture: A robust PF3 deployment requires a clean, dry gas supply line with redundancy, high-purity filters, and moisture control. Inline gas purifiers and moisture sensors help maintain process integrity. The delivery system should minimize residence time and avoid cold spots that could condense the gas.
- Process chamber considerations: Materials compatibility is critical in process chambers. PF3 can be corrosive to certain materials; selecting chamber materials and seals compatible with fluorinated gases minimizes degradation and contamination risk. Temperature control around the reaction zone helps manage reactivity.
- Contamination control: In ultra-high-purity environments, trace impurities can drastically alter film properties or etch profiles. Implement rigorous QC checks for PF3 batches, including impurity assays and certificate-of-analysis reviews. Use of bake-out procedures and surface preconditioning can reduce adsorption issues.
- Process monitoring: Real-time gas composition monitoring near the point of use helps detect impurities and drift in PF3 quality. In-situ sensors and chromatography-based analysis provide data to adjust process parameters promptly, ensuring reproducibility.
7. Practical usage guidelines and decision criteria
- When to select PF3: Consider PF3 when a fluorinated surface layer with precise, controllable characteristics is required, and when other fluorinated precursors do not deliver the necessary reactivity or selectivity. PF3 is particularly valuable in tight process windows where small changes in fluorination can have outsized effects on film attributes.
- Dose optimization: Determine the appropriate PF3 exposure by correlating gas flow, process time, and substrate response. This may involve calibration runs and design-of-experiments (DOE) to map etch rate, roughness, and film composition against PF3 delivery profiles.
- Compatibility checks: Before integration, assess the compatibility of PF3 with existing gas lines, regulators, and process chambers. Evaluate potential interactions with upstream chemistries and downstream cleaning steps to avoid cross-contamination or adverse reactions.
- Maintenance cadence: Schedule regular maintenance for seals, regulators, and purifiers, especially in systems operating with fluorinated gases. Periodic recalibration of MFCs ensures reliable delivery and process reproducibility.
8. Detailed product information: parameters, performance, and usage notes
- Typical specifications:
- Purity: ≥99.9% (with trace impurity profiles defined by supplier)
- Moisture content: < 1–5 ppm (varies by grade and supplier)
- Oxygen content: < 1–2 ppm
- Storage pressure: Cylinders rated for standard gas pressure with appropriate pressure relief devices
- Performance benchmarks:
- Consistent flow at specified MFC settings across operational temperature ranges
- Minimal adsorption/desorption losses in compatible lines
- Predictable fluorination activity under controlled humidity and temperature
- Usage notes:
- Pre-condition gas lines to remove residual moisture before introducing PF3
- Use dry, inert gas purging during setup and maintenance
- Implement inline scrubbers capable of handling hydrolysis byproducts
- Verify compatibility of PF3 with downstream materials to prevent corrosion and contamination
9. Environmental and regulatory considerations
- Environmental impact: While PF3 itself is valuable for precise processing, responsible usage includes containment, recovery, and appropriate disposal of spent gas or byproducts. Gas recovery systems can reclaim PF3 for reuse, reducing waste and environmental footprint.
- Regulatory landscape: Ensure compliance with local and international standards governing handling, transport, and use of fluorinated gases. Training, record-keeping, and workplace safety protocols should align with occupational safety guidelines and environmental regulations.
- Sustainability opportunities: Process optimization that reduces PF3 consumption without compromising performance can yield cost savings and lower emissions. Exploring alternative gas chemistries for specific steps may offer additional sustainability benefits.
10. Conclusion: PF3 as a cornerstone for future-ready processing
Phosphorus trifluoride embodies the blend of precise chemistry, robust engineering, and disciplined safety practices required for next-generation material processing. When integrated thoughtfully, PF3 enables refined surface modification, controlled fluorination, and reliable process reproducibility that collectively drive higher yields, improved device performance, and innovative materials architectures. As the industry advances toward ever-smaller feature sizes and increasingly complex material stacks, PF3’s role as a targeted fluorinating agent and surface-chemistry enabler is poised to expand, guided by rigorous process design, meticulous gas handling, and a steadfast commitment to safety and quality.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )