A Comprehensive Guide to Drawing the Lewis Structure of Phosphorus Trifluoride (PF3): From Valence Electrons to VSEPR
BY STEVEN, Published August 20, 2025
Phosphorus trifluoride (PF3) is a prototypical polar, covalent compound that serves as a valuable case study for applying Lewis structures, formal charge considerations, and the VSEPR model. This article provides a rigorous, practitioner-oriented walkthrough—from counting valence electrons and constructing an accurate Lewis structure to predicting molecular geometry with VSEPR, including practical notes on reactivity, handling, and safety considerations for PF3 and related fluoride gases. The discussion is designed to be accessible to educators, researchers, and industry professionals who work with rare and specialty gases, highlighting how electronic structure informs physical properties, reactivity, and application potential.
1. Fundamental electronic accounting: valence electrons and initial skeleton
- Valence electron count
- Phosphorus (P) is in group 15, contributing 5 valence electrons.
- Fluorine (F) is in group 17, contributing 7 valence electrons per atom.
- PF3 has one phosphorus atom and three fluorine atoms:
- Total valence electrons = 5 (P) + 3 × 7 (F) = 5 + 21 = 26 electrons.
- Constructing a skeletal structure
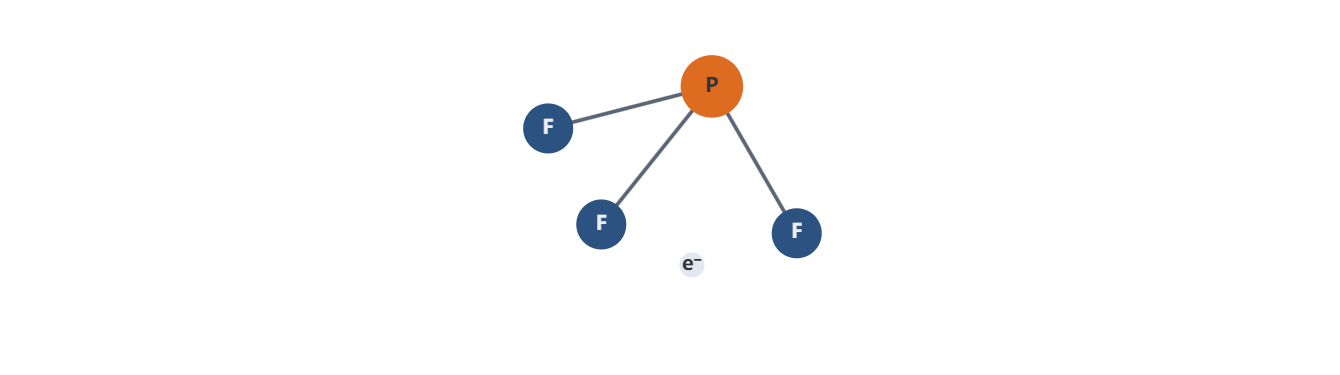
- The central atom is phosphorus, with three fluorine atoms forming single bonds to P, yielding a PF3 skeleton: F–P–F with one F remaining as a terminal substituent on each P–F bond.
- The initial Lewis structure places three P–F single bonds, using 6 electrons (3 bonds × 2 electrons per bond).
2. Completing octets and assessing formal charges
- Completing the octets
- After forming three P–F single bonds, phosphorus has 6 electrons in bonds, but electrons in bonds count as shared; each P–F bond counts as 2 electrons toward P’s valence count. To satisfy octets, phosphorus typically seeks 8 electrons in its valence shell.
- Among the remaining electrons: 26 total − (3 bonds × 2 electrons) = 20 electrons left to distribute as lone pairs.
- In a typical PF3 skeleton, fluorine atoms will receive three lone pairs each (3 × 6 = 18 electrons) for each F, totaling 54 electrons; this would exceed the available. The standard approach is:
- Allocate 6 electrons to each F as lone pairs (three lone pairs on each F) would be 3 × 6 = 18 electrons, but remember fluorine needs 3 lone pairs to complete its octet after the single P–F bond (7 valence electrons per F; with one bond shared, it needs 3 lone pairs = 6 electrons). This uses 3 × 6 = 18 electrons for lone pairs plus 3 bonds (6 electrons) = 24 electrons; we actually have 26 electrons, leaving 2 electrons to be placed as a lone pair on phosphorus, if following the simple octet rule. However, phosphorus in PF3 typically does not carry a full 8-electron octet; it forms three bonds and may have a lone pair, giving it a total of 10 electrons around phosphorus in some representations.
- Canonical Lewis structure approach
- The conventional representation for PF3 is with phosphorus in the center, three P–F single bonds, and a lone pair on phosphorus to reflect a total of 26 electrons: 3 P–F bonds (6 electrons) + 3×6 electrons for F lone pairs = 18 electrons + 2 electrons as a lone pair on P = 26 electrons.
- This yields: PF3 with a lone pair on P, and three bonded fluorines each with three lone pairs.
- Formal charge check
- P: Valence 5 − [nonbonding electrons on P (2) + 1/2 × bonding electrons (6)] = 5 − [2 + 3] = 0
- Each F: Valence 7 − [nonbonding electrons on F (6) + 1/2 × bonding electrons (2)] = 7 − [6 + 1] = 0
- All formal charges are zero, supporting this as a reasonable Lewis structure. The presence of a lone pair on P is consistent with phosphorus’s valence in PF3 and aligns with observed geometry.
3. Hybridization and electron-domain considerations
- Electron-domain geometry
- PF3 has four electron domains around the central phosphorus: three P–F bonding pairs and one lone pair. This suggests a tetrahedral electron-domain geometry.
- Hybridization
- The idealized hybridization for four electron domains is sp3. The three P–F bonds account for three bonding hybrids, and the lone pair occupies the fourth sp3 orbital.
- Implications for molecular shape
- The presence of the lone pair on phosphorus distorts the geometry from a perfect tetrahedron. According to VSEPR theory, PF3 adopts a trigonal pyramidal molecular geometry, analogous to ammonia (NH3), with the lone pair occupying one apex of the tetrahedron and the three P–F bonds forming the base of the pyramid.
4. VSEPR model: predicting geometry and bond angles
- Electron-domain vs. molecular geometry
- Electron-domain geometry: Tetrahedral (4 electron domains: 3 bonds + 1 lone pair)
- Molecular geometry: Trigonal pyramidal (three bonding pairs and one lone pair)
- Bond angle considerations
- In a perfect tetrahedron, bond angles are ~109.5°. However, lone pairs occupy more space than bonding pairs, compressing the bond angles between the P–F bonds slightly.
- In PF3, the F–P–F bond angles are slightly less than 109.5°, typically around 96–100° in similar trigonal pyramidal molecules, with exact values influenced by the lone pair repulsion and the electronegativity of the fluorine substituents.
- Dipole moment and polarity
- PF3 is polar due to the bent shape around phosphorus and the high electronegativity difference between P and F. The molecule exhibits a net dipole moment pointing from phosphorus toward the lone-pair region (roughly along the axis of the lone pair, but the exact vector is determined by charge distribution). This leads to strong interactions in condensed phases and notable reactivity in gas- and solution-phase processes.
5. Comparison with related species: PF5, PF2, and PF3 isomerism
- PF4− and PF5 differences
- PF5 would have phosphorus in an expanded octet with five P–F bonds in a trigonal bipyramidal arrangement. PF4− is a tetrahedral anion, often described as [PF4]− with a lone pair or hypervalent considerations.
- PF3 vs. PF3−
- PF3 is neutral with a lone pair on P; PF3− would have an extra electron, potentially altering geometry toward a distorted tetrahedral arrangement if the extra electron occupies a nonbonding orbital, illustrating how electron count governs structure.
- Hypervalency and resonance
- PF3 generally does not require d-orbital participation in bonding to an extent that overrides the classic Lewis and VSEPR framework. The main explanatory power comes from valence electron count, lone pair repulsion, and electronegativity-driven bond polarity. In heavier p-block fluorides, hypervalent explanations may be invoked, but the Lewis/VSEPR perspective remains robust for PF3 in most practical contexts.
6. Practical considerations for handling PF3 and related gases
- Properties and handling
- PF3 is a reactive, toxic, and corrosive gas under many conditions. It is typically handled in gas-handling systems designed for reactive fluorides, with materials compatibility considerations for fluorine-containing gases (e.g., nickel, Monel, certain fluoropolymer coatings). Proper ventilation, gas monitoring, and personal protective equipment (PPE) are essential in laboratory and industrial settings.
- In the gas phase, PF3’s reactivity is influenced by its electronic structure. The lone pair on phosphorus can participate in interactions with electrophiles or Lewis acids, impacting coordination chemistry and potential catalytic behavior in specific processes.
- Safety and regulatory notes
- Due to toxicity and corrosivity, PF3 handling requires dedicated gas cabinets, scrubbers, and emergency response plans. Waste streams must be managed according to applicable chemical waste regulations, with appropriate neutralization and containment strategies.
- Storage and transport
- Liquefied or compressed PF3 should be stored in containers compatible with fluorinated gases, with leak detection and pressure relief systems. Temperature and pressure controls are critical to maintain safe storage conditions and prevent decomposition or hazardous reactions.
7. Applications and implications of PF3’s structure
- Spectroscopic fingerprints
- The trigonal pyramidal geometry and the P–F bond characteristic frequencies yield distinct infrared (IR) absorption signals, with stretching modes influenced by bond strength and the presence of the lone pair. Nuclear magnetic resonance (NMR) visibility for fluorine-containing species often requires fluorine-specific instruments; phosphorus-31 NMR can reveal coupling patterns consistent with the PF3 framework.
- Reactivity patterns
- The electron lone pair on phosphorus enables PF3 to act as a Lewis base in adduct formation with strong Lewis acids. This behavior is relevant in catalytic contexts and in coordination chemistry studies involving rare gases and fluorinated ligands.
- Role in gas mixtures and synthesis
- PF3 may be used in specialty gas mixtures for calibration, semiconductor processing, or chemical vapor deposition (CVD) contexts where precise control of gas composition and reactivity is important. Its properties influence how it behaves in mixture with other fluorinated gases or with oxidants, depending on the intended application.
8. Structural visualization: Lewis structure to 3D geometry
- Step-by-step transformation
- Step 1: Draw P at the center with three single bonds to F, forming 3 P–F bonds.
- Step 2: Place a lone pair on P to account for the remaining electrons (total of 26 electrons in PF3).
- Step 3: Assign lone pairs to each F to complete their octets (three lone pairs on each F, resulting in 18 electrons for the F lone pairs).
- Step 4: Apply VSEPR to predict geometry: four electron domains around P (3 bonds, 1 lone pair) give a tetrahedral electron-domain geometry and a trigonal pyramidal molecular geometry.
- Step 5: Visualize the 3D shape with the lone pair occupying one apex, compressing the F–P–F angles and giving a pyramidal arrangement.
- Dimensional analysis and computational confirmation
- Modern computational chemistry methods (ab initio, DFT, or MP2) can reproduce the predicted geometry and provide bond lengths, angles, and vibrational frequencies that align with the observed physical properties of PF3 in gas and condensed phases.
9. Product parameters, performance metrics, and usage notes for PF3
- Key parameters
- Molecular formula: PF3
- Molar mass: approximately 100.99 g/mol
- Geometry: Trigonal pyramidal, with a central P atom bearing a lone pair
- Bond type: Predominantly polar covalent P–F bonds
- Dipole moment: Significant, due to bond polarity and molecular asymmetry
- Performance characteristics
- PF3’s reactivity profile is influenced by the phosphorous lone pair and the high electronegativity of fluorine, making it a moderate Lewis base and a potential ligand in coordination chemistry.
- The gas-phase behavior depends on pressure and temperature, with typical handling and storage requiring a controlled environment to prevent unwanted reactions with moisture or oxidants.
- Usage considerations
- When introducing PF3 into a system, ensure compatible materials and appropriate containment to prevent corrosion or toxic exposure. Use scrubbers and detectors for fluorinated gases, with established leak-testing procedures.
- In process gas streams for semiconductor fabrication or specialized chemistry labs, PF3 might be mixed with inert diluents or reactive partners, dependent on the target reaction pathway and system design.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )