PF3 Lewis Structure Explained: How Phosphorus Lone Pairs Tune Molecular Geometry
BY STEVEN, Published August 20, 2025
Phosphorus trifluoride (PF3) stands out among main-group compounds for its intriguing interplay between electronic structure and molecular geometry. In PF3, a phosphorus atom bonded to three fluorine atoms and bearing a lone pair reveals how lone-pair electrons influence shape, reactivity, and properties. This article provides a rigorous, expert-focused examination of the PF3 Lewis structure, the resulting geometry, and the factors that govern this system. We’ll also discuss practical considerations for handling PF3 in research and industrial settings, including safety, storage, and instrumentation considerations for precise measurements and analyses. The discussion blends fundamental valence-shell electron-pair repulsion (VSEPR) concepts with advanced orbital and spectroscopic insights, translating complex electronic structure into actionable understanding for specialists working with exotic and specialized gases.
1. Fundamental electron-counting and Lewis framework
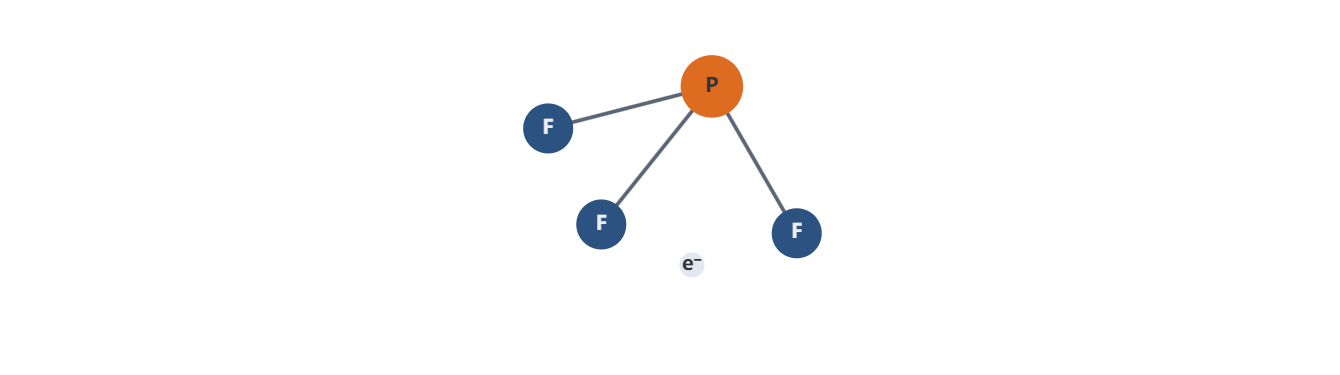
- Valence electrons: Phosphorus has five valence electrons, while each fluorine atom contributes seven. PF3 thus has a total of 5 + 3×7 = 26 valence electrons.
- Distribution: In the most conventional Lewis depiction, three P–F bonds account for six electrons per bond pair, contributing 6 electrons bonded, and a remaining pair (lone pair) on phosphorus accounts for two electrons.
- Initial sketch: PF3 can be envisioned as P with three single bonds to F atoms and one lone pair on P, giving the formal electron arrangement around P as three bonding domains and one lone pair.
2.VSEPR-based geometry and bonding considerations
- Electron-domain geometry: With three bonding pairs and one lone pair, the steric number around phosphorus is 4, suggesting a tetrahedral electron-domain geometry.
- Molecular geometry: The presence of one lone pair reduces the idealized bond-bond angles from 109.5° (tetrahedral) to slightly smaller values due to lone-pair–bond-pair repulsions. The resulting molecular geometry is trigonal pyramidal, with the lone pair occupying a quasi-apical position and the three P–F bonds forming the base of the pyramid.
- Bond angles: In PF3, the P–F–P angles are typically around 100–105°, reflecting the influence of the lone pair, though the exact value can vary with environment (e.g., solid-state packing, matrix effects, or interactions with solvents or coordinate ligands).
- Comparison with isostructural analogs: For example, PCl3 also shows a trigonal pyramidal geometry, illustrating the generality of lone-pair influence in phosphorus trihalides. PF3’s higher electronegativity of fluorine atoms modulates bond strengths and dipole interactions compared to heavier halides.
3. Electronic structure nuances: bonding, polarity, and lone-pair dynamics
- Bonding framework: PF3 features three P–F sigma bonds formed primarily by phosphorus 3p orbital overlap with fluorine 2p orbitals. The phosphorus center exhibits a relatively low-lying lone pair that is directional, contributing to the observed molecular shape.
- Lone pair character: The lone pair on phosphorus in PF3 is not a passive spectator. Its spatial orientation and energy affect the overall electron distribution, contributing to dipole moment and reactivity patterns. In the gas phase, the molecule exhibits a modest dipole moment due to the polar P–F bonds, oriented with the lone pair in a position that minimizes repulsion with the bonding electrons.
- Hybridization perspective: A common valence-bond interpretation treats phosphorus as using sp3 hybridization to accommodate three P–F bonds and one lone pair. However, modern computational approaches emphasize a more nuanced picture with contributions from p-character and hyperconjugative interactions, reinforcing that the lone pair is not purely localized in a single sp3 orbital but has a mixed orbital character.
4. Spectroscopic fingerprints and experimental validation
- Vibrational spectroscopy: PF3 exhibits characteristic vibrational modes corresponding to P–F stretching and bending motions. The presence of a lone pair influences the force constants and, therefore, the observed frequencies. Infrared (IR) spectroscopy typically reveals strong P–F stretches, while Raman activity may highlight symmetric and asymmetric stretches depending on selection rules.
- Rotational and electronic spectra: Microwave spectroscopy can probe rotational constants, yielding insight into molecular geometry in the gas phase. Ultraviolet-visible (UV-Vis) spectra are generally not highly structured for PF3 due to limited low-energy electronic transitions, but any weak transitions can inform about the electronic distribution around P.
- Computational corroboration: High-level ab initio and density functional theory (DFT) calculations reproduce the trigonal-pyramidal geometry, confirm the estimated dipole moment, and help quantify the lone-pair influence on bond lengths and angles. Such calculations also assist in interpreting how external fields, coordination to metals, or pressure variation modulates geometry.
5. Reactivity and coordination chemistry implications
- Electrophilicity and nucleophilicity: PF3 is a Lewis base at phosphorus due to its lone pair, yet the strong P–F bonds and the electron-withdrawing nature of fluorine modulate access to the phosphorus center. PF3 can donate electron density to metal centers in coordination complexes, acting as a ligand in organometallic chemistry. Conversely, the P lone pair can participate in non-covalent interactions, influencing conjunctions with Lewis acids.
- Coordination behavior: In complexes, PF3 can act as a σ-donor and, in some cases, as a π-acceptor ligand depending on the metal center and the electronic environment. The trigonal-pyramidal geometry around phosphorus can become distorted upon binding to metals or other acceptors, leading to changes in bond lengths and angles that reflect back on the ligand field and catalytic behavior.
- Reactivity under extreme conditions: As with many fluorinated species, PF3 can be reactive under high-energy conditions (e.g., photolysis, plasma environments). In such scenarios, ligand dissociation or fluorine transfer events can occur, and the lone pair may participate in rearrangements that affect the gas-phase chemistry and potential applications in plasma processing.
6. Practical considerations for handling PF3: safety, storage, and instrumentation
- Safety profile: PF3 is a highly reactive, toxic gas with a distinct odor that can be hazardous at low concentrations. It is necessary to operate under strict gas-handling protocols, including proper ventilation, leak detection, and appropriate personal protective equipment. Given the reactivity of fluorinated phosphorus species, monitoring for potential reactions with moisture or oxidizers is essential.
- Storage and containment: PF3 should be stored in compatible materials and under conditions that minimize decomposition or reaction with container materials. Perfluorinated elastomers, corrosion-resistant alloys, and suitable gasketing materials are often selected to tolerate fluorinated gas environments. Cylinders should be clearly labeled with hazard information and have pressure-relief devices calibrated for the specific gas mixture.
- Instrumentation and measurement: For accurate structural determinations and property measurements, techniques such as infrared spectroscopy, Raman spectroscopy, microwave spectroscopy, and electron diffraction can be employed. Gas-phase PF3 studies may utilize effusive beam sources for rotational spectroscopy and mass spectrometry for composition verification. Computational support ties experimental results to electronic structure explanations, reinforcing the reliability of the interpretation.
- Environmental and regulatory considerations: Fluorinated phosphorus species can have environmental implications. Compliance with transport, storage, and disposal regulations is mandatory, including proper capture and scrubbing strategies for accidental releases and adherence to regional guidelines on toxic gas management.
7. Parameter-rich product overview: PF3 as a research and industrial gas
- Physical properties: PF3 is a colorless to pale-yellow gas at room temperature with a distinctive pungent odor. It has a moderate vapor pressure and a relatively high reactivity toward moisture and strong oxidizers due to the polar P–F bonds.
- Purity and grades: PF3 is offered in various purities, often with stringent specifications for trace impurities that can influence reactivity, catalytic activity, or spectroscopic measurements. High-purity PF3 is commonly used in gas-phase coordination chemistry, gas-phase synthesis, and spectroscopic calibration.
- Typical applications: PF3 serves as a ligand in coordination chemistry studies, including metal–PF3 adducts, and as a reactive gas in fluorination processes under controlled conditions. It also acts as a model system for understanding lone-pair effects in phosphorus tri-halide chemistry, aiding in theoretical and computational comparisons across the group.
- Handling guidelines: Always operate PF3 within certified gas cabinets or gloveboxes designed for hazardous fluorinated gases. Use compatible regulators, tubing, and seals to prevent leaks. Employ calibrated gas detectors for real-time monitoring and ensure emergency response procedures are well understood by personnel.
8. The role of PF3 in teaching and research: lessons for geometry and electronic structure
- Conceptual clarity: PF3 offers a concrete example of how a lone pair modulates geometry in a three-body bond framework. It reaffirms the idea that bond angles are not fixed by simple tetrahedral assumptions but are tuned by repulsions from lone pairs.
- Experimental integration: PF3 demonstrates how spectroscopy and computational chemistry complement one another. Students and researchers can compare measured vibrational frequencies and rotational constants with predicted values to validate electronic structure models.
- Cross-disciplinary relevance: The PF3 case bridges inorganic chemistry, organometallic chemistry, materials science, and gas-phase spectroscopy, illustrating how fundamental concepts translate into real-world laboratory practice.
9. Frequently encountered questions and clarifications
- Why is PF3 not perfectly tetrahedral? Because the lone pair on phosphorus exerts greater repulsion than bonding pairs, compressing the P–F–P angles and yielding a trigonal-pyramidal geometry.
- Does PF3 have a significant dipole moment? Yes, attributable to the polar P–F bonds and the asymmetric distribution created by the lone pair. The dipole moment is a measurable indicator of the molecule’s polarity.
- Can PF3 act as a ligand? Indeed, PF3 behaves as a Lewis base at phosphorus and can coordinate to metal centers, influencing catalytic activity and electronic properties of the resulting complexes.
- How does environment affect geometry? Solvation, matrix effects, coordination to metals, pressure, and temperature changes can slightly alter bond lengths and angles due to shifts in electron density and repulsion dynamics.
10. Conclusion: synthesis of structure–property relationships in PF3
PF3 exemplifies how a single lone pair on a central phosphorus atom reshapes molecular geometry and reactivity in a system dominated by strong P–F bonds. The trigonal-pyramidal arrangement emerges from a delicate balance of electron repulsion, orbital interactions, and the polar nature of the P–F bonds. This geometry not only dictates physical properties such as dipole moment and vibrational characteristics but also governs PF3’s behavior as a ligand and its role in coordination chemistry. For researchers and practitioners, understanding PF3’s structural framework enhances our ability to predict reactivity patterns, tailor gas-phase experiments, and design novel compounds that leverage lone-pair effects for targeted applications in catalysis, materials science, and advanced gas-phase synthesis.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )