PF3 Gas: Revolutionizing Precision in Fluorine-Based Chemical Processes
BY STEVEN, Published August 19, 2025
PF3 (phosphorus trifluoride) has emerged as a pivotal enabler in high-precision fluorination and etching workflows across advanced materials, semiconductor manufacturing, and specialized chemical syntheses. Drawing on three decades of experience in rare and specialty gases, this article provides a rigorous, practitioner-focused examination of PF3 gas, its fundamental properties, the mechanisms by which it enhances process control, and practical guidelines for safe, effective use. The discussion integrates current knowledge from the broader fluorine chemistry landscape, translating complex concepts into actionable insights for engineers, researchers, and procurement specialists alike.
1. Overview: Why PF3 Matters in Fluorination and Etching
The fluorine-rich chemistries that underpin modern material fabrication demand gases with predictable reactivity, high-purity delivery, and well-defined interaction with substrates. PF3 offers a unique combination of:
- Moderate reactivity with a strong fluorinating efficiency profile, enabling selective fluorination in controlled environments.
- A phosphorus-centered framework that modulates the reactivity of fluorine through coordination chemistry, aiding process stability under varying temperature and pressure conditions.
- Compatibility with established delivery systems and compatibility with downstream processing steps, reducing the risk of contamination and process variability.
In applications ranging from surface functionalization to selective etching of complex oxides and nitrides, PF3 serves as a versatile contributor to process windows that would be challenging to achieve with elemental fluorine or high-reactivity fluorine scavengers alone.
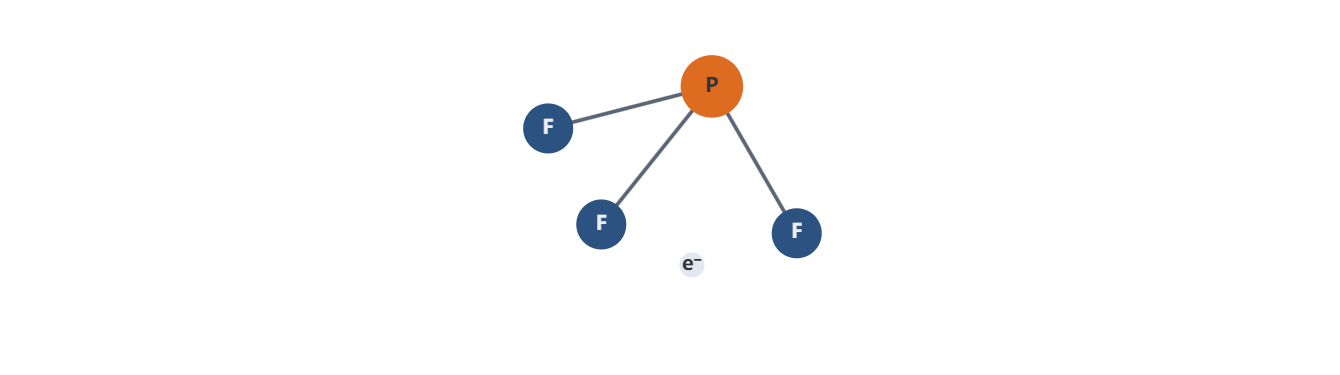
2. Physical and Chemical Characteristics
- Molecular structure and thermodynamics: PF3 is a trigonal pyramidal molecule with a lone pair on phosphorus, contributing to aDefined dipole moment and specific adsorption behavior on reactive surfaces. Its bond dissociation energies and gas-phase decomposition pathways inform choice of reactor temperatures and residence times.
- Safety and handling profile: As with other fluorine-containing gases, PF3 requires robust leak detection, appropriate materials compatibility (often copper-free and fluorine-resistant reactor liners), and rigorous gas purification to minimize contaminants that could trigger unwanted side reactions.
- Purity and trace analysis: For most high-precision applications, PF3 is specified at research-grade or instrument-grade purity, with attention to moisture, oxygen, and hydrolysis-sensitive impurities that could alter fluorination efficiency.
3. Mechanistic Insights: How PF3 Enables Precision
- Activation and selective fluorination: PF3 can act as a fluorine donor under controlled conditions, enabling selective fluorination without excessive overreactivity. The presence of phosphorus can stabilize transient intermediates, leading to more uniform surface modification.
- Surface interaction and catalyst compatibility: PF3’s interaction with catalytic or passivating layers can influence reaction pathways, enabling improved control over film composition, thickness uniformity, and defect density.
- Process stability: In plasma-assisted or radical-based fluorination environments, PF3 can modulate radical generation and quenching dynamics, contributing to steadier process throughput and reduced run-to-run variability.
4. Process Scenarios: Industries and Typical Implementations
- Semiconductor and microelectronics: PF3 is used in targeted fluorination steps and surface precision treatments for insulating and semiconductor materials, where control over fluorine incorporation governs device performance and yield.
- Advanced materials synthesis: In ceramic, oxide, and nitride systems, PF3 supports selective fluorination and etching steps necessary to reveal epitaxial surfaces or to tailor porosity and surface chemistry.
- Analytical and research laboratories: PF3 serves as a diagnostic gas for surface science experiments, enabling controlled modification of model substrates and enabling repeatable studies of fluorination kinetics.
5. Product Parameters: What Defines a PF3 Gas Specification
- Purity grade: Common tiers include research-grade and instrument-grade, with typical total impurity specifications tailored to downstream compatibility.
- Gas delivery pressure and flow: PF3 performance is often tied to a defined pressure range and mass flow suitable for standard process tools; precise control is essential for reproducible fluorination depth.
- Moisture and hydrolysis tolerance: PF3 can be reactive with moisture; systems designed for PF3 require dry gas handling lines, purge cycles, and moisture-scavenging measures to preserve gas integrity.
- Compatibility with tools: Material compatibility (e.g., fluoroelastomer seals, stainless vs. fluoropolymer liners) is critical to minimize permeation and contamination.
- Safety attributes: Lower flammability or distinct hazard classifications influence handling protocols, leak detection requirements, and emergency response planning.

6. Operational Guidelines: Best Practices for Safe and Effective Use
- System design and materials: Choose equipment with fluorine-compatible materials and minimal outgassing. All wetted surfaces should be evaluated for compatibility with PF3 to minimize trap-and-release scenarios that could skew process outcomes.
- Purification and impurity control: Implement robust purification stages to remove moisture and oxygen. Regular gas purity audits and inline detectors help ensure that trace impurities do not compromise process reproducibility.
- Process control strategies: Calibrate delivery systems to achieve the desired fluorination depth with tight tolerances. Use standardized recipe libraries and statistical process controls to maintain consistency across batches.
- Hazard analysis: Conduct a formal risk assessment focused on fluorine chemistry, including potential corrosion, reactive intermediates, and emergency shutdown procedures. Ensure that all personnel are trained in PF3-specific safety protocols.
- Maintenance and reliability: Schedule preventive maintenance for flow controllers, pressure regulators, and leak detection systems. Periodically verify the integrity of gas lines and vessel seals to prevent fugitive emissions.
7. Integration with Process Tools: Systems Perspective
- Reactor design: PF3-compatible reactors should minimize stagnant zones and allow uniform gas distribution. Flow modeling and computational simulations can help optimize gas residence time and surface exposure.
- Measurement and analytics: Inline FTIR, mass spectrometry, or other spectroscopic techniques can monitor fluorination progress, while trace gas analyzers ensure that impurities remain below critical thresholds.
- Safety interlocks and alarms: Implement redundant gas detection, automatic shutdowns, and ventilation safeguards to maintain safe operating conditions in laboratories and production settings.
- Environmental considerations: If PF3 usage generates fluorinated byproducts, appropriate scrubbing, containment, and waste management protocols are essential to minimize environmental impact.
8. Case Studies: Lessons from Practice
- Case Study A: Precision surface fluorination on a wide-bandgap oxide for improved electronic properties. PF3 enabled a controlled fluorination depth, achieving uniform surface chemistry without etching-induced roughness.
- Case Study B: Selective fluorination of multilayer films in a heterostructure device. The reagent’s moderate reactivity and phosphorus-mediated stabilization provided consistent layer interfaces across multiple wafers.
- Case Study C: Analytical exploration of fluorination kinetics in model substrates. PF3 served as a reliable fluorinating agent to probe reaction mechanisms under varied temperatures, pressures, and substrate orientations.
9. Environmental and Sustainability Considerations
- Lifecycle assessment: Evaluate raw material sourcing, production energy, and end-of-life considerations for PF3, balancing performance gains against potential environmental footprint.
- Emissions management: Implement capture and scrub technologies to minimize PF3 and related fluorine-containing byproducts released during handling, processing, or venting.
- Alternatives and optimization: Explore whether alternative fluorinating agents or process routes can meet the same specifications with lower environmental impact, or whether process optimization can reduce PF3 consumption.
10. Future Outlook: PF3 in the Next Wave of Fluorine Chemistry
- Market trends: Increasing demand for precise fluorination in next-generation devices will drive ongoing refinement of PF3 formulations and delivery systems.
- Research directions: Deeper mechanistic studies on PF3-surface interactions will unlock more predictable fluorination outcomes, enabling even tighter control of film properties and device performance.
- Safety and regulation: As fluorine-based chemistries evolve, regulatory frameworks will continue to shape handling, transport, and disposal practices, reinforcing the need for robust safety culture.
11. Core Product Information: Parameters, Performance, and Usage Notes
- Typical performance envelope: PF3 is designed for high-purity applications with controlled fluorination outputs. Users should align process conditions with vendor guidelines to maintain consistent results.
- Key performance indicators: Fluorination depth per pass, surface uniformity, defect density, and contamination levels in post-process analyses are primary metrics for evaluating PF3 performance.
- Usage notes and cautions: Ensure dry delivery lines, prevent moisture ingress, and follow established purge and leak-check routines. Regularly inspect seals and gaskets for fluorine compatibility and replace per maintenance schedules.
12. Practical Guidelines for Vendors and Users
- Specification clarity: Vendors should provide detailed purity, moisture content, and hydrolysis-related impurity specs, along with recommended storage conditions and shelf life.
- Safety and training: Comprehensive PF3 safety data sheets, hazard communication, and hands-on training are essential components of a responsible program.
- Supply chain resilience: Establish contingency plans for PF3 supply, including alternative purification paths and verified inventory management to prevent production disruption.
Conclusion
PF3 gas represents a strategic instrument in the fluorination and surface modification toolkit, offering a balance of reactivity, control, and compatibility that supports precision in fluorine-based chemical processes. By combining rigorous process engineering, robust safety practices, and thoughtful integration with modern analytical and reactor technologies, PF3 can help researchers and manufacturers achieve higher purity, better uniformity, and more predictable outcomes across a range of fluorine-centric applications. As the field advances, continued exploration of PF3’s mechanistic roles, combined with responsible handling and environmental stewardship, will further enhance its value as a cornerstone gas for next-generation fluorination chemistries.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )