Unveiling PF3: The Power of Phosphorus Trifluoride in Semiconductor Manufacturing
BY STEVEN, Published August 19, 2025
Phosphorus Trifluoride (PF3) stands as a cornerstone in the realm of specialty gases, driving innovation in industries where precision and purity are paramount. As a highly reactive, colorless, and odorless gas, PF3 has become indispensable in semiconductor manufacturing, offering unique chemical properties that enable the production of advanced microelectronics. With decades of expertise in specialty gases, this article explores the science, applications, and transformative potential of PF3, providing a comprehensive guide for professionals navigating the complexities of modern manufacturing.
Understanding Phosphorus Trifluoride (PF3)
Phosphorus Trifluoride (PF3) is a chemical compound featuring one phosphorus atom bonded to three fluorine atoms, forming a trigonal pyramidal structure due to a lone pair of electrons on the phosphorus. This molecular geometry underpins its reactivity, making it a versatile tool in high-tech applications. PF3 is produced through the controlled reaction of phosphorus trichloride (PCl3) with a fluoride source, such as hydrogen fluoride (HF), followed by rigorous purification to achieve the ultra-high purity levels demanded by industries like electronics.
The gas’s polar nature and reactivity with water, metals, and oxidizing agents necessitate specialized handling and storage protocols. Its role in semiconductor manufacturing, however, hinges on its ability to deliver phosphorus atoms with precision, enabling the creation of high-performance electronic components.
Key Chemical and Physical Properties
To appreciate PF3’s role in industrial applications, it’s essential to understand its core properties:
-
Molecular Formula: PF3
-
Molecular Weight: 87.97 g/mol
-
Boiling Point: -101.8°C (-151.2°F)
-
Density: 3.91 g/L at standard temperature and pressure (STP)
-
Reactivity: Highly reactive with water, forming phosphoric and hydrofluoric acids; incompatible with strong oxidizers
-
Toxicity: Toxic upon inhalation, requiring stringent safety measures
-
Appearance: Colorless, odorless gas at ambient conditions
These properties make PF3 a specialized gas tailored for applications where molecular precision and purity are non-negotiable.
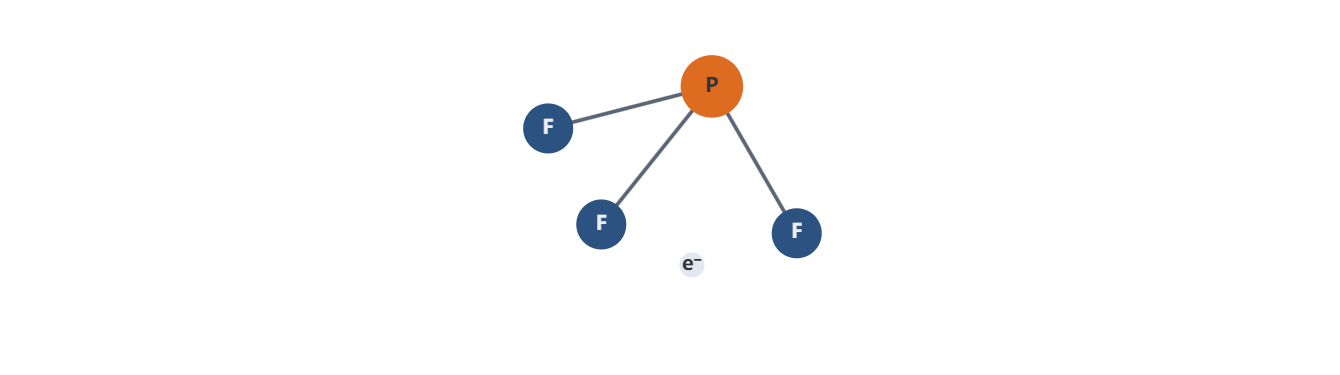
Phosphorus Trifluoride PF3 Lewis Structure
The Role of PF3 in Semiconductor Manufacturing
Semiconductor manufacturing is a cornerstone of modern technology, powering everything from smartphones to artificial intelligence systems. PF3 plays a pivotal role in this industry, particularly in doping and plasma etching processes, where its unique properties enhance efficiency and performance.
Phosphorus Doping for N-Type Semiconductors
In semiconductor fabrication, doping is the process of introducing impurities into silicon to alter its electrical properties. PF3 serves as a critical phosphorus source for creating n-type semiconductors, where phosphorus atoms donate free electrons to enhance conductivity. This is essential for producing components like transistors, diodes, and integrated circuits.
The high purity of PF3—often exceeding 99.999%—ensures that no unwanted impurities compromise the silicon wafer’s integrity. By delivering phosphorus atoms with precision, PF3 enables manufacturers to achieve the exact doping profiles required for advanced microchips, supporting the relentless push toward smaller, faster, and more efficient electronics.
Plasma Etching and Surface Modification
PF3 is also employed in plasma etching, a process used to create intricate patterns on semiconductor surfaces. In this application, PF3 is ionized in a plasma environment to form reactive species that selectively remove material from the wafer. The gas’s ability to produce volatile byproducts ensures clean etching, minimizing residue and improving yield. This is particularly critical in the production of nanoscale features, where precision is measured in angstroms.
Beyond Semiconductors: Emerging Applications
While semiconductors are the primary domain of PF3, its utility extends to other high-tech fields:
-
Chemical Synthesis: PF3 acts as a precursor in the production of fluorophosphates, used in flame retardants, battery electrolytes, and pharmaceutical intermediates.
-
Coordination Chemistry: In research settings, PF3 serves as a ligand, forming complexes with transition metals for catalytic studies.
-
Gas Mixtures: PF3 is blended with inert gases like argon or nitrogen for specialized applications, such as calibration standards or gas-phase deposition.
Technical Advantages of PF3
PF3’s prominence in semiconductor manufacturing stems from its distinct technical advantages, which set it apart from other doping and etching gases:
-
Ultra-High Purity: Available in grades up to 99.99%, PF3 minimizes contamination risks in cleanroom environments.
-
Selective Reactivity: Its controlled reactivity ensures precise chemical interactions, reducing unwanted byproducts.
-
Compatibility: PF3 integrates seamlessly with existing gas delivery systems, simplifying adoption in manufacturing workflows.
-
Efficiency: The gas’s volatility facilitates the removal of reaction byproducts, enhancing process cleanliness and yield.
-
Versatility: Beyond doping, PF3’s applications in etching and chemical synthesis make it a multi-purpose tool.
These advantages position PF3 as a critical enabler of precision and performance in high-stakes industries.
Product Specifications and Handling Guidelines
For professionals working with PF3, a thorough understanding of its technical specifications and safety protocols is essential. Below is a detailed overview of the product’s parameters and handling requirements:
|
Parameter |
Details |
|---|---|
|
Purity |
≥99.99% or higher for semiconductor applications |
|
Cylinder Sizes |
Available in 10L, 40L, 47L, or custom high-pressure cylinders |
|
Pressure |
Supplied at 1000–1500 psig (69–103 bar) |
|
Storage Conditions |
Store in a cool, dry, well-ventilated area, away from heat and oxidizers |
|
Material Compatibility |
Use with stainless steel or fluoropolymer-lined regulators and piping |
|
Safety Equipment |
Gas detectors, ventilation systems, and personal protective equipment (PPE) |
Handling and Safety Precautions
Given PF3’s toxicity and reactivity, strict adherence to safety protocols is critical:
-
Ventilation: Use PF3 in a fume hood or well-ventilated area to prevent inhalation exposure.
-
Personal Protective Equipment (PPE): Wear chemical-resistant gloves, safety goggles, and respiratory protection.
-
Emergency Response: In case of a leak, evacuate the area, deploy gas detectors, and follow established emergency protocols.
-
Storage: Secure cylinders upright, away from incompatible materials like water or oxidizing agents.
-
Disposal: Neutralize and dispose of residual gas through certified hazardous waste facilities to prevent environmental harm.
Safety and Regulatory Compliance
PF3 is classified as a toxic and corrosive substance under global regulations, such as the Globally Harmonized System (GHS). Key safety and regulatory considerations include:
-
Inhalation Hazards: Exposure to PF3 can cause severe respiratory irritation or damage. Adhere to occupational exposure limits, such as those set by OSHA or ACGIH.
-
Reactivity Risks: Contact with water or oxidizers can produce hazardous byproducts, including hydrofluoric acid, which is highly corrosive.
-
Regulatory Standards: Compliance with regulations like OSHA (United States), REACH (European Union), or local equivalents is mandatory for safe handling, transport, and disposal.
-
Training: Personnel must be trained in PF3’s properties, risks, and emergency procedures, with access to up-to-date safety data sheets (SDS).
Optimizing PF3 in Semiconductor Workflows
To maximize PF3’s benefits, manufacturers must adopt best practices for its integration into production processes. Below are actionable strategies:
-
Precision Gas Delivery: Use high-precision mass flow controllers to regulate PF3 flow, ensuring consistent doping and etching results.
-
Real-Time Monitoring: Deploy gas analyzers to detect impurities, safeguarding process integrity in cleanroom environments.
-
Exhaust Management: Implement scrubbers and neutralization systems to handle PF3 byproducts, minimizing environmental impact.
-
Staff Training: Conduct regular training on PF3 handling, safety, and emergency response to maintain a secure workplace.
-
Supplier Partnerships: Collaborate with reputable gas suppliers to ensure consistent PF3 quality and reliable delivery schedules.
The Future of PF3 in Semiconductor Innovation
The semiconductor industry is at a pivotal moment, driven by the demand for smaller, faster, and more energy-efficient chips. PF3 is poised to play a central role in this evolution, supporting the development of next-generation technologies like 3nm and 2nm nodes. Its precision in doping and etching aligns with the industry’s push for nanoscale accuracy, enabling the production of advanced processors for artificial intelligence, 5G, and quantum computing.
Beyond semiconductors, PF3’s applications in fluorinated compound synthesis are gaining traction in emerging fields like lithium-ion battery electrolytes and sustainable materials. As industries prioritize efficiency and sustainability, PF3’s versatility and compatibility with green manufacturing processes will drive its adoption.
Technological advancements, such as AI-driven process optimization, are also enhancing PF3’s utility. AI systems can fine-tune gas delivery, predict equipment maintenance, and optimize doping profiles, further improving yield and reducing costs. As these trends converge, PF3 will remain a linchpin in the specialty gas ecosystem.
PF3’s Broader Impact on High-Tech Industries
The influence of PF3 extends beyond its immediate applications, shaping the broader landscape of high-tech manufacturing. Its role in enabling smaller, more powerful electronics supports advancements in consumer devices, medical equipment, and renewable energy systems. By facilitating the production of high-performance semiconductors, PF3 contributes to global innovation, powering technologies that define the modern era.
Moreover, the gas’s applications in chemical synthesis are opening new frontiers in materials science. Fluorophosphates derived from PF3 are being explored for use in solid-state batteries, which promise higher energy densities and safety for electric vehicles. These developments underscore PF3’s potential to drive progress across multiple sectors.
Best Practices for Sourcing and Using PF3
For organizations integrating PF3 into their operations, strategic sourcing and handling are critical. Key recommendations include:
-
Select Reputable Suppliers: Partner with suppliers offering ultra-high-purity PF3 and robust quality assurance processes.
-
Validate Purity: Request certificates of analysis (CoA) to confirm PF3 meets the required purity standards for your application.
-
Invest in Infrastructure: Equip facilities with compatible gas delivery systems, ventilation, and monitoring equipment to ensure safety and efficiency.
-
Stay Regulatory-Compliant: Regularly review and adhere to local and international regulations governing PF3 use and disposal.
By prioritizing these practices, manufacturers can harness PF3’s full potential while maintaining safety and operational excellence.
Phosphorus Trifluoride (PF3) is more than a specialty gas—it is a catalyst for precision and innovation in semiconductor manufacturing and beyond. Its unique properties, from ultra-high purity to selective reactivity, make it an essential tool for producing the microchips that power modern technology. By understanding its applications, technical advantages, and safety requirements, industries can leverage PF3 to achieve unparalleled performance and efficiency.
As the demand for advanced electronics and sustainable materials grows, PF3 will continue to play a pivotal role in shaping the future of high-tech manufacturing. For professionals seeking to stay ahead in this dynamic field, mastering the use of PF3 is not just an opportunity—it’s a necessity.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )