Propyne Formula and Structure: The Science Behind a Key Specialty Gas
BY STEVEN, Published August 17, 2025
Propyne, also known as methylacetylene, is a specialty hydrocarbon gas with the chemical formula C₃H₄. As a member of the alkyne family, its distinctive carbon-carbon triple bond makes it a vital component in industrial processes, organic synthesis, and cutting-edge scientific research. With applications ranging from high-temperature welding to rocket propulsion and astrophysical studies, propyne’s unique chemical structure and reactive properties position it as a cornerstone in the specialty gas sector. This article delves into the science behind propyne’s formula and structure, exploring its chemical properties, applications, and practical considerations for researchers and industry professionals. Drawing on decades of expertise in nuclear elements and specialty gases, the following sections provide a comprehensive, accessible, and authoritative overview of propyne’s role in advancing science and technology.
1. The Chemistry of Propyne: Decoding C₃H₄
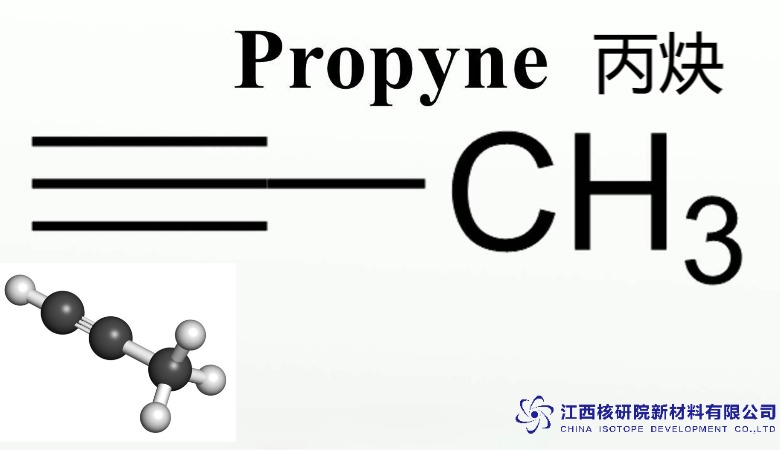
Propyne, with the molecular formula C₃H₄, is the second simplest alkyne after acetylene (C₂H₂). Its chemical structure, CH₃C≡CH, features a three-carbon chain with a triple bond between the second and third carbon atoms. This triple bond, consisting of one sigma bond and two pi bonds, is the hallmark of alkynes, conferring high electron density and reactivity. Propyne’s compact molecular weight of 40.06 g/mol and gaseous state at room temperature make it a versatile compound for both industrial and laboratory applications.
The physical properties of propyne enhance its utility across diverse settings. With a boiling point of -23.2°C (249.95 K) and a melting point of -102.7°C (170.45 K), propyne exists as a colorless gas with a sweet, characteristic odor at standard conditions. Its low solubility in water (3,640 mg/L at 20°C) contrasts with its high solubility in organic solvents like ethanol, chloroform, and benzene, making it an ideal candidate for organic synthesis. Propyne’s flammability, with explosive limits of 1.7% to 11.7% in air, underscores the need for stringent safety protocols during handling and storage.
2. Structural Insights: The Power of the Triple Bond
The molecular structure of propyne is defined by its linear arrangement around the carbon-carbon triple bond. The structure, H₃C-C≡CH, can be broken down as follows:
- Carbon 1 (CH₃): A methyl group bonded to three hydrogens and the second carbon via a single sigma bond.
- Carbon 2 (C≡): Triple-bonded to the third carbon and single-bonded to the first carbon, contributing to the molecule’s rigidity.
- Carbon 3 (≡CH): Triple-bonded to the second carbon and single-bonded to a hydrogen atom, known as the alkynyl hydrogen.
This configuration results in a bond angle of approximately 180° around the triple-bonded carbons, giving propyne a linear geometry in that region. The triple bond’s high electron density makes it highly reactive, particularly in addition reactions where electrophiles or nucleophiles target the pi electrons. For example, deprotonation of the alkynyl hydrogen with a strong base like n-butyllithium forms propynyllithium, a nucleophilic reagent widely used in organic synthesis.
In nuclear magnetic resonance (NMR) spectroscopy, propyne exhibits a characteristic singlet at approximately 1.8 ppm in deuteriochloroform, reflecting the overlapping signals of its propargylic and alkynyl protons. This spectral signature aids in its identification and analysis in chemical research. The triple bond also contributes to propyne’s high energy content, with a heat of combustion of approximately 1,937 kJ/mol, making it a potent fuel for high-temperature applications.
3. Key Applications of Propyne in Industry and Research
Propyne’s reactivity and physical properties enable a broad spectrum of applications, from industrial processes to cutting-edge scientific investigations. Below are the primary domains where propyne excels:
- Welding and Metalworking: Propyne is a key component of MAPP gas (Methylacetylene-Propadiene Propane), a fuel mixture used in oxy-fuel welding and cutting. Its high flame temperature, approaching 2,900°C in oxygen, makes it ideal for brazing, soldering, and cutting metals. Unlike acetylene, propyne can be safely condensed, reducing the risk of explosive decomposition during storage.
- Organic Synthesis: As a three-carbon building block, propyne is invaluable in organic chemistry. Its alkynyl hydrogen can be deprotonated to form reactive intermediates, such as propynyllithium, which reacts with carbonyl compounds to produce alcohols, esters, and other complex molecules. Propyne is also used in the synthesis of alkylated hydroquinones, a critical step in producing vitamin E and other pharmaceuticals.
- Rocket Propulsion: Research, particularly in Europe, has explored propyne as a liquid rocket propellant when combined with liquid oxygen. Its specific impulse of approximately 370 seconds, high density (0.6070 g/cm³ as a liquid), and moderate boiling point make it a less toxic alternative to traditional propellants like monomethylhydrazine (MMH). Propyne’s condensability simplifies storage compared to cryogenic fuels, enhancing its suitability for space missions.
- Astrochemistry: Propyne has been detected in interstellar clouds, such as the Sgr B2 molecular cloud, and in the atmospheres of gas giants like Jupiter, Saturn, Uranus, and Neptune. Its presence provides insights into the formation of polycyclic aromatic hydrocarbons (PAHs), which are precursors to complex organic molecules in space. Propyne’s spectral signatures, observable via infrared and radio astronomy, are critical for studying molecular evolution in the cosmos.
- Material Science: Propyne serves as a starting material for producing synthetic rubber, resins, and advanced polymers. Its reactivity in polymerization reactions supports the development of high-performance materials used in coatings, adhesives, and composites.
4. Technical Advantages of Propyne as a Specialty Gas
Propyne’s unique properties confer several technical advantages, making it a preferred choice in specific applications:
- High Reactivity: The triple bond enables propyne to participate in diverse chemical reactions, from addition to nucleophilic substitutions, enhancing its utility in synthesis.
- Condensability: Unlike acetylene, propyne can be safely liquefied and stored under pressure, reducing safety risks in industrial applications.
- High Energy Content: Its heat of combustion supports its use as a high-performance fuel in welding and propulsion systems.
- Analytical Utility: Propyne’s distinct NMR and infrared spectral signatures facilitate precise identification and analysis in research settings.
- Astrophysical Relevance: Its detection in interstellar and planetary environments underscores its importance in studying cosmic chemistry.
5. Product Specifications and Practical Considerations
To provide a comprehensive understanding of propyne for researchers and industry professionals, the following table outlines its key specifications and performance characteristics:
| Parameter | Specification |
|---|---|
| Chemical Formula | C₃H₄ (CH₃C≡CH) |
| Molecular Weight | 40.06 g/mol |
| Appearance | Colorless gas with a sweet odor |
| Boiling Point | -23.2°C (249.95 K) |
| Melting Point | -102.7°C (170.45 K) |
| Density | 0.6070 g/cm³ (liquid at boiling point); 1.41 (vapor, relative to air) |
| Solubility | 3,640 mg/L in water at 20°C; highly soluble in ethanol, chloroform, benzene |
| Vapor Pressure | 5.2 atm at 20°C |
| Explosive Limits | 1.7%–11.7% in air |
| Flash Point | -51°C |
| Purity Level | ≥99.5% (suitable for industrial and research applications) |
| Exposure Limits | NIOSH REL: TWA 1,000 ppm (1,650 mg/m³); IDLH: 1,700 ppm |
| Storage Form | Liquefied compressed gas in high-pressure cylinders (e.g., 25 g, 100 g, 500 g) |
Performance Characteristics:
- Flammability: Highly flammable, requiring explosion-proof equipment and strict ignition control.
- Reactivity: Forms explosive acetylides with silver, copper, and mercury salts; incompatible with copper alloys (>65% Cu), Monel, neoprene, and polyethylene.
- Stability: Stable under controlled conditions but may form explosive peroxides during prolonged storage, necessitating regular inspection.
- Spectral Properties: NMR singlet at 1.8 ppm in deuteriochloroform; infrared absorption at 2,135 cm⁻¹ (C≡C stretch).
Usage Considerations:
- Storage: Store in high-pressure cylinders in a cool, well-ventilated area, away from heat sources, oxidizers, and ignition sources. Use explosion-proof fittings and comply with OSHA 1910.101 standards.
- Handling: Use gas-handling systems with adequate ventilation to prevent asphyxiation or flash fires. Employ PPE, including respirators, in high-concentration environments.
- Safety: Moderately toxic by inhalation; high concentrations may cause dizziness, narcosis, or asphyxiation. Avoid contact with incompatible materials to prevent explosive reactions.
- Disposal: Return unused cylinders to suppliers or incinerate in a chemical incinerator equipped with an afterburner and scrubber, adhering to environmental regulations.
6. Practical Implementation in Research and Industry
In industrial applications, propyne is typically delivered as a liquefied compressed gas in high-pressure cylinders. For welding, it is blended with propadiene and propane to form MAPP gas, which is fed into oxy-fuel torches via regulators to produce high-temperature flames. In organic synthesis, propyne is handled in controlled reactors under inert atmospheres (e.g., nitrogen or argon) to prevent oxidation. Catalysts or strong bases are used to activate its triple bond for reactions like alkylation or addition.
In research settings, propyne is analyzed using gas chromatography-mass spectrometry (GC-MS) or NMR spectroscopy to study reaction mechanisms or isotopic variants (e.g., propyne-d4). In astrophysical studies, high-purity propyne is used to calibrate infrared and radio telescopes, enabling the detection of its spectral signatures in interstellar clouds or planetary atmospheres. Laboratories must maintain strict safety protocols, including regular inspection of cylinders for peroxide formation, to mitigate explosion risks.
For rocket propulsion, propyne is stored in specialized tanks designed to handle its moderate boiling point and high vapor pressure. Its integration into propulsion systems requires precise control of fuel-oxidizer ratios to optimize combustion efficiency. Research facilities, such as those in the European Space Agency, use propyne in test engines to evaluate its performance against traditional propellants.
7. Environmental and Safety Considerations
Propyne’s high volatility contributes to its environmental impact, as it can react with atmospheric nitrogen oxides to form ground-level ozone and particulate matter, which are linked to respiratory issues and climate change. Industrial facilities must employ scrubbers and emission control systems to comply with regulations like the U.S. Clean Air Act or EU air quality standards. Propyne’s flammability and potential to form explosive peroxides necessitate robust safety measures, including explosion-proof storage areas, fire suppression systems, and trained personnel.
Inhalation exposure should be limited to 1,000 ppm (TWA), as higher concentrations may cause central nervous system effects, including dizziness or unconsciousness. Proper ventilation and gas detection systems are critical in confined spaces. Additionally, propyne’s incompatibility with certain metals and polymers requires careful selection of materials for storage and handling systems to prevent hazardous reactions.
8. The Future of Propyne in Science and Technology
The future of propyne is bright, driven by its versatility and potential in emerging fields. In material science, researchers are exploring propyne’s role in synthesizing novel polymers and nanomaterials, leveraging its reactive triple bond to create high-performance coatings and composites. In pharmaceuticals, propyne’s use as a synthetic intermediate could lead to new drug molecules, particularly in the development of antioxidants and anti-inflammatory compounds.
In aerospace, propyne’s potential as a greener rocket propellant is gaining traction. Its lower toxicity compared to hydrazine-based fuels aligns with global efforts to reduce the environmental impact of space exploration. Ongoing research aims to optimize propyne’s combustion characteristics and storage systems for use in low Earth orbit and deep-space missions.
In astrochemistry, propyne’s detection in interstellar environments continues to inform models of molecular evolution and the origins of life. Advances in spectroscopic techniques and space-based observatories, such as the James Webb Space Telescope, will enhance our understanding of propyne’s role in forming complex organic molecules. However, challenges remain, including the energy-intensive production of propyne via propane cracking and the need for sustainable synthesis methods to meet growing demand.
9. Strategic Importance in the Specialty Gas Market
Propyne’s production is limited by the complexity of its synthesis, primarily through thermal or catalytic pyrolysis of propene, a byproduct of petroleum refining. Its high cost as a purified gas drives interest in using MAPP gas as a cost-effective source for industrial applications. Global suppliers must navigate supply chain constraints, including energy costs and regulatory compliance, to ensure a stable supply. Strategic investments in catalytic technologies and recycling systems could enhance propyne’s accessibility and reduce its environmental footprint.
The specialty gas market, valued at billions annually, is driven by demand for high-purity gases in electronics, aerospace, and research. Propyne’s niche role in this market underscores its importance as a high-value commodity. Partnerships between gas suppliers, research institutions, and industrial manufacturers will be critical to scaling production and expanding applications.
10.Propyne’s Role in Advancing Science and Industry
Propyne (C₃H₄) is a specialty gas with far-reaching implications, bridging industrial utility and scientific discovery. Its triple bond structure, high reactivity, and condensability make it indispensable in welding, organic synthesis, rocket propulsion, and astrophysical research. By understanding its chemical properties, optimizing its handling, and exploring its potential in emerging fields, researchers and industry professionals can unlock new opportunities for innovation. As technology evolves and global challenges like sustainability and space exploration take center stage, propyne will remain a key player in shaping the future of science and industry.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )