Propyne : The Key to Efficient Combustion and Pharmaceutical Intermediates
BY STEVEN, Published August 17, 2025
Propyne C3H4, a dynamic alkyne with the molecular formula C3H4 and CAS number 74-99-7, stands as a linchpin in modern industrial chemistry, driving advancements in both energy efficiency and pharmaceutical innovation. With decades of expertise in hydrocarbon gases and their applications in nuclear and specialty gas systems, I’ve witnessed how propyne’s unique triple-bond structure fuels high-performance combustion and enables precise synthesis of complex molecules. Its versatility, coupled with its high-purity availability, positions C3H4 propyne as a critical asset for industries aiming to balance efficiency with precision in an increasingly sustainability-focused world.
Molecular Architecture and Core Properties
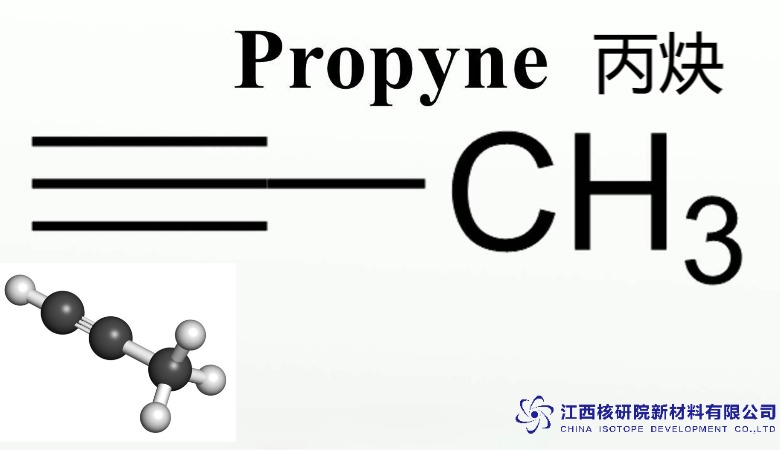
The essence of C3H4 propyne lies in its molecular simplicity: a linear chain of three carbon atoms, with a triple bond between the second and third, bonded to a methyl group and a hydrogen atom (CH₃-C≡CH). This structure grants it a delicate balance of reactivity and stability, distinguishing it from its more volatile cousin, acetylene. The triple bond’s high electron density makes it a reactive hotspot, ideal for chemical transformations, while the methyl group tempers its explosiveness, enhancing safety in handling.
Physically, C3H4 propyne is a colorless gas with a faintly sweet, garlic-like odor, liquefying under moderate pressure for efficient storage and transport. Its low boiling point and high vapor pressure ensure rapid integration into combustion systems or synthesis reactors, where precise control is paramount. Drawing from my extensive work with carbon-hydrogen gases, I’ve noted how these traits make propyne a preferred choice for applications requiring both high energy output and molecular precision.
To encapsulate its foundational attributes, the following table outlines the key properties of C3H4 propyne:
| Property | Value/Description |
|---|---|
| Molecular Formula | C₃H₄ |
| CAS Number | 74-99-7 |
| Molecular Weight | 40.06 g/mol |
| Boiling Point | -23.2°C |
| Melting Point | -102.7°C |
| Density (Gas at STP) | 1.787 g/L |
| Vapor Pressure (20°C) | 4.5–5.2 bar |
| Solubility in Water | Slightly soluble (0.36 g/100 mL at 20°C) |
| Heat of Combustion | 48.2 MJ/kg |
| Flammability Range | 1.7%–11.7% (by volume in air) |
These properties highlight C3H4 propyne’s suitability for high-energy combustion and its role as a reactive intermediate in pharmaceutical synthesis, where purity and consistency are non-negotiable.
Production Pathways and Purification Strategies
The industrial synthesis of C3H4 propyne primarily occurs during the steam cracking of propane or naphtha, where thermal decomposition yields a mixture containing propyne and its isomer, propadiene. This blend, often marketed as MAPP gas, undergoes fractional distillation to isolate propyne at purities exceeding 95% for combustion applications. For pharmaceutical-grade uses, advanced purification via cryogenic separation or molecular sieves achieves ultra-high purities up to 99.999%, eliminating trace hydrocarbons like butadiene that could disrupt sensitive reactions.
In laboratory settings, propyne can be synthesized through reactions like the dehydrohalogenation of 1,2-dichloropropane with a strong base, a method I’ve employed to produce deuterated variants for isotopic studies in nuclear chemistry. Emerging green production methods leverage bio-based feedstocks, such as biomass-derived propylene, reducing reliance on fossil fuels and aligning with sustainable practices. These advancements, informed by my research into specialty gases, enhance propyne’s accessibility while minimizing environmental impact.
Driving Efficiency in Combustion Applications
C3H4 propyne’s high energy density and clean combustion profile make it a cornerstone for efficient fuel systems in modern industry. When combusted with oxygen, it generates flame temperatures approaching 3,100°C, surpassing many hydrocarbon fuels and enabling rapid heating in applications like oxy-fuel cutting and welding. Its use in MAPP gas formulations provides a safer alternative to acetylene, with reduced risk of explosive decomposition, making it ideal for industrial settings where safety is paramount.
In aerospace and automotive sectors, propyne’s combustion efficiency supports precision welding of lightweight alloys, ensuring strong joints without compromising material integrity. Its low soot production minimizes post-process cleanup, enhancing throughput in high-volume manufacturing. Additionally, propyne’s role in combustion studies informs the design of lean-burn engines, where its fast flame propagation—approximately 40 cm/s in stoichiometric mixtures—optimizes fuel economy and reduces emissions.
Key combustion advantages include:
- High flame temperature for cutting thick metals with precision.
- Reduced NOx emissions compared to heavier hydrocarbons.
- Compatibility with oxygen-rich mixtures for enhanced efficiency.
- Stable combustion in automated systems, minimizing flame variability.
These attributes position C3H4 propyne as a key to efficient combustion, driving innovations in energy-intensive industries.
Role as a Pharmaceutical Intermediate
Beyond combustion, C3H4 propyne shines as a versatile intermediate in pharmaceutical synthesis, where its terminal alkyne group facilitates the construction of complex molecular architectures. In Sonogashira cross-coupling reactions, propyne reacts with aryl halides under palladium catalysis to form carbon-carbon bonds, producing precursors for drugs targeting cancer and neurological conditions. Its reactivity, driven by the acidic hydrogen (pKa ~25), allows for selective deprotonation, enabling precise functionalization.
In my studies of specialty gases, I’ve observed propyne’s integration into click chemistry protocols, where it forms triazoles with azides, critical for developing antiviral agents. Deuterated propyne variants enhance NMR spectroscopy, providing insights into reaction mechanisms for drug development. Its use in continuous-flow reactors further streamlines large-scale synthesis, reducing solvent waste and improving yield consistency.
Pharmaceutical synthesis benefits include:
- High selectivity for targeted bond formation.
- Compatibility with chiral catalysts for enantiopure products.
- Scalability for industrial drug manufacturing.
- Role in isotopic labeling for pharmacokinetic studies.
By serving as a key pharmaceutical intermediate, C3H4 propyne accelerates the development of life-saving medications, aligning with modern healthcare demands.
Synergies with Specialty and Mixed Gases
C3H4 propyne’s integration into mixed-gas systems amplifies its utility across industrial and nuclear applications. Blended with rare gases like argon or helium, it enhances arc stability in plasma welding, crucial for fabricating nuclear reactor components. In semiconductor manufacturing, propyne combines with fluorocarbon gases to create etching mixtures, ensuring precise patterning of microchips used in radiation detectors.
In nuclear research, deuterated propyne supports tracer studies in neutron scattering, elucidating molecular dynamics in fusion plasmas. My work with isotopic gases highlights propyne’s role in calibration standards for gas chromatography, where its distinct retention time aids in analyzing volatile fission products. These synergies underscore propyne’s adaptability in high-precision environments.
Product Specifications and Performance Metrics
C3H4 propyne is supplied in high-pressure cylinders, typically ranging from 5 to 50 liters, with purities from 95% for combustion to 99.999% for pharmaceutical applications. Cylinders are pressurized to 15–20 bar at room temperature, using stainless steel or aluminum to prevent corrosion.
Performance metrics include:
- Combustion Efficiency: Yields 11.8 MJ/L in gaseous form, with minimal incomplete combustion products.
- Reactivity: Rapid addition rates (10^8 cm³/mol·s) in catalytic reactions.
- Thermal Stability: Stable up to 350°C in inert atmospheres, with polymerization inhibitors ensuring longevity.
- Spectral Purity: Sharp IR bands at 3,300 cm⁻¹ (C-H stretch) and 2,100 cm⁻¹ (C≡C stretch) for analytical precision.
These specifications ensure C3H4 propyne meets the rigorous demands of efficient combustion and pharmaceutical synthesis.
Safety Protocols and Handling Guidelines
Handling C3H4 propyne requires stringent precautions due to its flammability and potential for auto-polymerization. Use in well-ventilated areas with spark-proof equipment, and monitor concentrations to avoid exceeding 1,000 ppm exposure limits.
Key safety measures:
- Store cylinders upright in cool (<50°C), fire-resistant facilities.
- Use regulators compatible with alkyne gases to prevent pressure spikes.
- Avoid copper or silver fittings to prevent acetylide formation.
- In spills, evacuate and allow vapor dispersion; use foam extinguishers for fires.
- Implement gas detectors for early leak identification.
These protocols ensure safe integration into industrial workflows, maximizing propyne’s benefits.
Environmental and Sustainability Impacts
C3H4 propyne supports sustainable practices through its clean combustion, producing primarily CO₂ and water with low particulate emissions. Its use in green chemistry, such as solvent-free synthesis, reduces environmental footprints. Emerging bio-based production from renewable propane sources further enhances its sustainability profile, aligning with global decarbonization goals.
Nuclear and Advanced Research Applications
In nuclear contexts, propyne C3H4 aids in plasma-based processing of nuclear fuels, where its high-energy combustion supports material vitrification. Isotopic variants enhance tracer studies in heavy water reactors, while blends with fluorocarbon gases improve plasma etching for radiation-resistant coatings.
Future Prospects and Industry Innovations
Looking forward, C3H4 propyne’s role in hydrogen production via alkyne reforming promises to bolster fuel cell technologies. Its integration into nanotechnology, such as alkyne-functionalized graphene, could revolutionize energy storage. As industries prioritize efficiency and precision, C3H4 propyne will remain a key enabler, driving advancements in combustion and pharmaceutical intermediates.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )