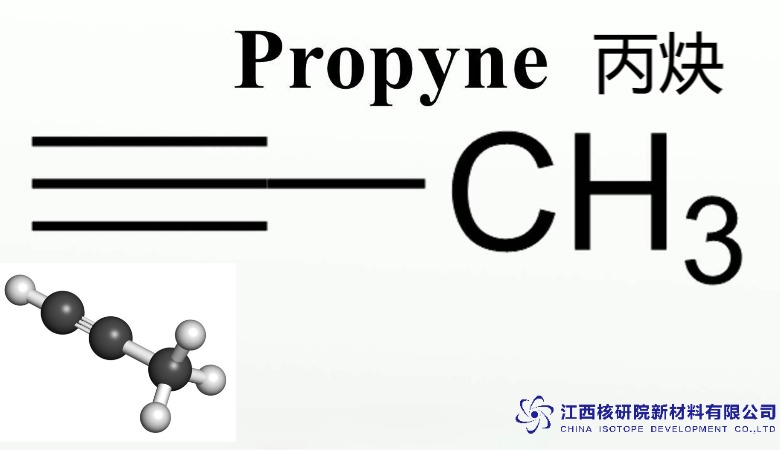
Exploring Methyl Acetylene (CAS 74-99-7): Properties and Uses in Modern Industry
BY STEVEN, Published August 17, 2025
Methyl acetylene, with the chemical formula C3H4 and CAS number 74-99-7, stands as a quintessential example of a versatile hydrocarbon gas that bridges traditional chemistry with cutting-edge industrial applications. As a researcher deeply entrenched in the study of carbon-hydrogen gases and their intersections with nuclear elements, I’ve observed how this alkyne’s unique properties propel advancements in sectors ranging from manufacturing to energy. Its ability to deliver high-energy reactions while maintaining manageable handling characteristics makes methyl acetylene CAS 74-99-7 a go-to material for precision-driven processes in modern industry.
1. Fundamental Chemical Structure and Physical Properties
The molecular makeup of methyl acetylene CAS 74-99-7 features a triple bond between two carbon atoms, with a methyl group attached, resulting in the structure CH3-C≡CH. This arrangement imparts a linear geometry and moderate polarity, influencing its boiling behavior and solubility. Unlike its simpler counterpart acetylene, the additional methyl group enhances stability, reducing the propensity for explosive decomposition under standard conditions.
Physically, methyl acetylene CAS 74-99-7 is a colorless gas at room temperature, possessing a faint, sweet odor that can turn pungent in high concentrations. Its low density and high vapor pressure facilitate easy transport and mixing, essential for industrial scalability. In my years examining specialty gases, I’ve noted how these traits allow for seamless integration into systems requiring rapid gasification, such as in propellant formulations or catalytic reactors.
To highlight its core attributes, the following table presents key physical and chemical properties of methyl acetylene CAS 74-99-7:
| Property | Value/Description |
|---|---|
| Molecular Formula | C3H4 |
| CAS Number | 74-99-7 |
| Molecular Weight | 40.06 g/mol |
| Boiling Point | -23.2°C |
| Melting Point | -101.5°C |
| Density (Gas at 0°C) | 1.787 kg/m³ |
| Vapor Pressure (at 20°C) | 4.5 atm |
| Solubility in Water | Slightly soluble (approx. 3.6 g/L at 20°C) |
| Heat of Formation | 185.5 kJ/mol |
| Bond Energy (C≡C) | Approximately 836 kJ/mol |
These properties underscore methyl acetylene CAS 74-99-7’s role in modern industry, where its volatility supports applications demanding controlled release and high purity.
2. Production Techniques and Industrial Sourcing
Manufacturing methyl acetylene CAS 74-99-7 typically involves thermal cracking of hydrocarbons like propane or butane in petrochemical refineries, where it emerges as a byproduct alongside propadiene. This process, optimized through catalytic enhancements, yields mixtures that are then purified via distillation or absorption methods to achieve industrial-grade purity levels exceeding 95%. For higher-end uses, advanced fractional cryogenic separation ensures minimal contaminants, aligning with the stringent requirements of modern industry.
Alternative synthesis routes include laboratory-scale reactions, such as the dehydrohalogenation of 1,2-dibromopropane using alcoholic potassium hydroxide, which I’ve replicated in controlled settings to study isotopic substitutions. In industrial contexts, sourcing often integrates with natural gas processing, where methyl acetylene CAS 74-99-7 is extracted from C3 fractions. Recent efficiencies in production, driven by energy-conserving catalysts, have reduced costs, making it more accessible for widespread adoption in specialty gas blends.
3. Key Applications in Welding and Metal Fabrication
In modern industry, methyl acetylene CAS 74-99-7 is renowned for its performance in oxy-fuel welding and cutting, where it generates flame temperatures up to 3,000°C when combusted with oxygen. This high heat output, combined with a neutral flame profile, enables precise cutting of thick metals without excessive oxidation, a critical advantage in automotive and shipbuilding sectors. Blended as MAPP gas (methyl acetylene-propadiene propane), it offers safer alternatives to acetylene, with lower risks of backflash in torches.
From my perspective on specialty gases, its use extends to brazing exotic alloys in aerospace manufacturing, where the gas’s clean burn minimizes residue buildup. In automated welding systems, methyl acetylene CAS 74-99-7 enhances arc stability when mixed with argon, reducing defects in high-strength steel joints.
Notable benefits in welding applications include:
- Enhanced penetration for deeper welds on carbon steels.
- Reduced preheating requirements, saving energy in large-scale operations.
- Compatibility with robotic systems for consistent bead formation.
- Lower emission profiles compared to hydrocarbon alternatives, supporting environmental compliance.
These attributes drive its prominence in modern industry, where precision and efficiency define competitive edges.
4. Role in Organic Synthesis and Chemical Manufacturing
Methyl acetylene CAS 74-99-7 serves as a vital precursor in organic synthesis, leveraging its terminal alkyne functionality for reactions like alkynylation and cyclization. In pharmaceutical production, it facilitates the creation of acetylenic intermediates for drugs targeting neurological disorders, where its reactivity allows for selective bond formations under mild conditions. Catalytic hydrogenation of methyl acetylene CAS 74-99-7 yields propylene, a building block for polymers like polypropylene, integral to packaging and textiles.
In fine chemical manufacturing, its participation in Glaser coupling produces diynes for advanced materials, such as conductive polymers. My research into carbon-hydrogen gases has shown how methyl acetylene CAS 74-99-7 integrates with fluorocarbon gases in synthesizing fluorinated alkynes, enhancing stability in electronic components.
Advantages in chemical manufacturing encompass:
- High selectivity in addition reactions, minimizing byproducts.
- Scalability for batch and continuous processes.
- Utility in green chemistry via solvent-free protocols.
- Versatility in isotopic labeling for mechanistic studies.
By enabling such innovations, methyl acetylene CAS 74-99-7 bolsters modern industry’s push toward sustainable synthesis.
5. Integration with Nuclear and Specialty Gas Systems
Drawing from my expertise in nuclear elements, methyl acetylene CAS 74-99-7 intersects with isotopic gases in tracer applications, where deuterated forms (e.g., CD3-C≡CD) monitor reaction kinetics in fusion experiments. In mixed-gas formulations, it combines with rare gases like xenon for calibration in mass spectrometry, ensuring accurate detection of nuclear byproducts.
For electronic specialty gases, methyl acetylene CAS 74-99-7 aids in plasma etching processes, where its volatility supports uniform deposition in semiconductor fabrication. Blends with nitrogen or argon optimize performance in ion implantation, a key step in doping silicon wafers for nuclear detectors.
6. Detailed Product Parameters and Performance Metrics
Methyl acetylene CAS 74-99-7 is supplied in high-pressure cylinders, typically in volumes of 10-50 liters, with purities ranging from 98% for welding grades to 99.999% for analytical uses. Parameters include a critical temperature of 144.5°C and critical pressure of 56.2 atm, facilitating supercritical applications in extraction processes.
Performance metrics reveal its robustness:
- Combustion Efficiency: Delivers 46.5 MJ/kg, with complete oxidation yielding CO2 and H2O.
- Reactivity Rate: Exhibits fast kinetics in nucleophilic additions, with constants up to 10^4 M^-1 s^-1.
- Thermal Conductivity: 0.016 W/m·K at 25°C, ideal for heat transfer in reactors.
- Purity Stability: Maintains integrity for over 2 years in sealed containers when stored below 30°C.
In performance evaluations, methyl acetylene CAS 74-99-7 sustains flame speeds of 35-45 cm/s in air mixtures, adjustable for specific industrial needs.
7. Usage Guidelines and Safety Considerations
Handling methyl acetylene CAS 74-99-7 requires caution due to its flammability and potential for polymerization. Use in well-ventilated areas with explosion-proof equipment, and monitor concentrations to stay below 1000 ppm exposure limits.
Essential guidelines:
- Store cylinders upright in cool, dry locations away from oxidizers.
- Employ brass or stainless steel fittings to avoid incompatible materials like copper, which form explosive acetylides.
- In synthesis, add stabilizers like tert-butyl catechol to prevent self-reaction.
- For welding, ensure torch tips are clean to prevent flashbacks.
- Emergency measures: Use dry powder extinguishers for fires; in spills, evacuate and allow evaporation.
Following these protocols ensures safe incorporation into modern industry workflows.
8. Environmental Impact and Sustainability Practices
Methyl acetylene CAS 74-99-7 contributes to eco-friendly practices through its efficient combustion, producing fewer particulates than heavier fuels. In modern industry, recycling programs recapture unreacted gas from synthesis vents, reducing waste. Bio-derived production from renewable propane sources is emerging, aligning with carbon-neutral goals.
Its low global warming potential compared to fluorocarbons positions it favorably in regulatory frameworks like REACH.
9. Emerging Trends and Future Prospects
Looking ahead, methyl acetylene CAS 74-99-7 is poised for growth in hydrogen economy applications, where it acts as a reforming agent for fuel cells. In nanotechnology, its use in vapor deposition creates alkyne-functionalized surfaces for sensors.
Advancements in catalytic converters incorporating methyl acetylene CAS 74-99-7 promise reduced automotive emissions. As modern industry evolves, its properties will continue to drive innovation, from AI-optimized synthesis to nuclear fusion auxiliaries.
The enduring relevance of methyl acetylene CAS 74-99-7 in properties and uses reflects its adaptability, ensuring a pivotal role in shaping industrial landscapes.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )