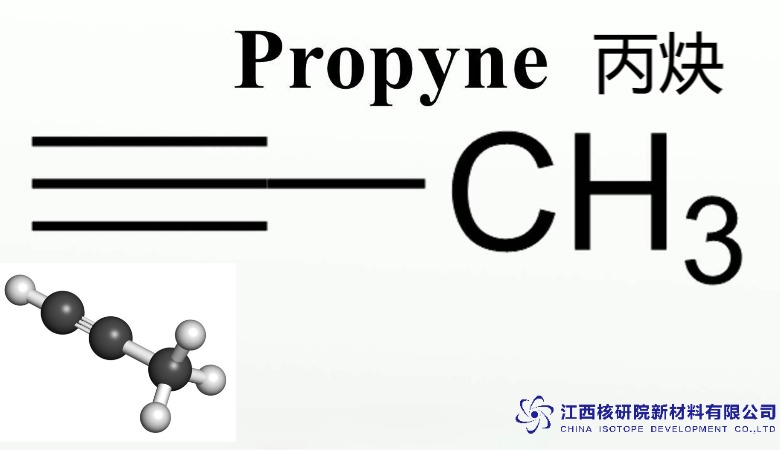
Propyne C3H4 Gas: Driving Precision in Gas Welding and Organic Synthesis
BY STEVEN, Published August 17, 2025
Propyne C3H4 gas, a lightweight alkyne with remarkable versatility, has long been a staple in my explorations of carbon-hydrogen gases within the broader nuclear and specialty gas domains. Over decades of research, I’ve seen how its triple bond structure enables exacting control in high-stakes processes, from the fine-tuned flames of gas welding to the intricate pathways of organic synthesis. This gas not only enhances precision but also integrates seamlessly with mixed gases and isotopic variants, offering solutions that align with modern demands for efficiency and sustainability in chemical engineering.
1. Core Structure and Physicochemical Profile
At the heart of propyne C3H4 gas lies a compact molecular framework: three carbon atoms aligned with a triple bond between the second and third, attached to a methyl group and a terminal hydrogen (CH₃-C≡CH). This configuration grants it a unique blend of stability and reactivity, setting it apart from simpler alkynes like acetylene. The triple bond’s electron density facilitates nucleophilic attacks, while the methyl substituent moderates volatility, making propyne C3H4 gas easier to handle in precision applications.
Physically, propyne C3H4 appears as a colorless, flammable gas with a mild ethereal odor, condensing into a liquid under moderate pressure. Its low boiling point and high vapor pressure allow for precise delivery in welding torches or synthesis reactors, where flow rates must be meticulously controlled. In my studies of hydrocarbon gases, I’ve noted how these traits contribute to its role in driving precision, particularly when blended with rare gases like argon for enhanced arc stability.
To provide a clear snapshot, here’s a table summarizing the essential properties of propyne C3H4 gas:
| Property | Value/Description |
|---|---|
| Molecular Formula | C₃H₄ |
| Molecular Weight | 40.06 g/mol |
| Boiling Point | -23.2°C |
| Melting Point | -102.7°C |
| Density (Gas at STP) | 1.787 g/L |
| Vapor Pressure (20°C) | Approximately 5.2 bar |
| Solubility in Water | Low (about 0.36 g/100 mL at 20°C) |
| Heat of Combustion | 48.2 MJ/kg |
| Autoignition Temperature | 428°C |
| Flammability Limits in Air | 1.7% – 11.7% vol |
These parameters highlight propyne C3H4 gas’s suitability for environments requiring rapid phase changes and controlled energy release, foundational to its precision-driven uses.
2. Synthesis Routes and Refinement Processes
The production of propyne C3H4 gas draws from established petrochemical techniques, refined over years to meet the purity demands of gas welding and organic synthesis. Primarily, it arises as a co-product in the steam cracking of propane, where high temperatures cleave bonds to form a mixture including propyne and its isomer, allene. Separation via fractional distillation yields high-purity streams, often exceeding 98% for welding-grade gas.
Alternative laboratory methods include the base-catalyzed elimination from 1,2-dihalopropanes or the alkylation of acetylene with methyl halides, allowing for customized synthesis when isotopic labeling is needed—such as incorporating deuterium for tracer studies in nuclear reactions. Refinement involves cryogenic purification to remove impurities like propene, ensuring the gas’s integrity for precision tasks. In specialty contexts, propyne C3H4 gas is often stabilized with additives to prevent auto-polymerization, extending shelf life in cylinders.
My experience with carbon-hydrogen gases reveals that catalytic hydrogenation steps can fine-tune isomer ratios, optimizing propyne C3H4 for specific welding flames or synthesis yields. These processes not only drive precision but also support scalable production, integrating with sustainable feedstocks like bio-propane for eco-friendly variants.
3. Precision Applications in Gas Welding
Propyne C3H4 gas excels in gas welding by delivering focused, high-temperature flames that enable intricate metal joining with minimal distortion. When mixed with oxygen in formulations like MAPP gas (methylacetylene-propadiene propane), it produces temperatures up to 2,900°C, surpassing propane while offering safer handling than acetylene due to lower explosion risks. This precision is vital for welding thin-gauge metals in aerospace components, where even slight overheating can compromise structural integrity.
In oxy-fuel cutting, propyne C3H4 gas’s clean combustion reduces slag formation, allowing for smoother edges on stainless steel or aluminum. Welders appreciate its stable flame envelope, which maintains consistency across varying tip sizes and pressures. Blending with argon or helium further enhances arc welding processes, such as TIG, by improving shielding and reducing oxidation—key for precision in nuclear vessel fabrication.
Advantages in gas welding include:
- Superior heat concentration for detailed work on alloys.
- Reduced preheating times, boosting productivity.
- Compatibility with automated systems for repeatable results.
- Lower soot production, minimizing post-weld cleanup.
These features position propyne C3H4 gas as a driver of precision, particularly in industries where weld quality directly impacts safety and performance.
4. Advancements in Organic Synthesis
In organic synthesis, propyne C3H4 gas serves as a reactive building block, enabling the construction of complex molecules with atomic-level accuracy. Its terminal alkyne group participates in click chemistry reactions, such as copper-catalyzed azide-alkyne cycloadditions, forming triazoles for pharmaceutical scaffolds. This precision is crucial in synthesizing APIs where stereochemistry must be preserved.
Propyne C3H4 gas also facilitates hydroboration-oxidation sequences, yielding alcohols or aldehydes with high regioselectivity. In my research on hydrocarbon gases, I’ve seen its utility in cross-coupling with halides via palladium catalysis, creating conjugated systems for materials like OLEDs. When deuterated, it aids in mechanistic studies, tracing pathways in nuclear magnetic resonance spectroscopy.
Key benefits for organic synthesis encompass:
- High reactivity for efficient, low-waste reactions.
- Versatility in functional group transformations.
- Integration with flow reactors for continuous precision synthesis.
- Role in asymmetric catalysis for chiral molecule production.
By driving such precision, propyne C3H4 gas accelerates innovation in drug discovery and materials science, where molecular exactitude defines success.
5. Synergies with Mixed and Specialty Gases
Propyne C3H4 gas’s true potential unfolds when incorporated into mixed gases, enhancing precision across welding and synthesis. Combined with fluorocarbons, it forms etching mixtures for semiconductor fabrication, where controlled volatility ensures uniform patterning. In nuclear applications, blending with rare gases like krypton provides calibration standards for gas chromatography, vital for analyzing fission products.
For welding, mixtures with carbon dioxide stabilize arcs in MIG processes, reducing spatter for cleaner joins. In synthesis, propyne C3H4 with nitrogen diluents moderates exothermic reactions, preventing runaway conditions in large-scale operations. These synergies extend to isotopic gases, where propyne variants support heavy water-based deuteration, linking to my expertise in fluorocarbon and deuterium reagents.
6. Product Specifications and Performance Analysis
Propyne C3H4 gas is commercially available in compressed cylinders, with purities from 95% for general welding to 99.99% for synthesis-grade applications. Cylinder capacities range from 10 to 50 kg, pressurized to 15-20 bar, often with valve outlets compliant to CGA standards.
Performance highlights include:
- Flame Characteristics: Achieves adiabatic flame temperatures of 3,100 K with oxygen, ideal for precision cuts.
- Reaction Kinetics: Exhibits rate constants around 10^8 cm³/mol·s in radical additions, ensuring swift synthesis steps.
- Thermal Stability: Withstands up to 300°C without decomposition in inert environments.
- Purity Impact: Impurities below 50 ppm maintain flame neutrality, preventing color shifts in welds.
In testing, propyne C3H4 demonstrates laminar flame speeds of 40-50 cm/s, adjustable via mixture ratios for optimized welding or synthesis conditions.
7. Handling Protocols and Safety Measures
Safe utilization of propyne C3H4 gas is paramount, given its flammability and potential for peroxide formation. Store in ventilated, non-sparking areas, securing cylinders against tipping. Use regulators rated for alkyne gases to avoid pressure surges.
Essential precautions:
- Monitor for leaks using electronic detectors; avoid open flames.
- Wear PPE including flame-retardant gloves and respirators in confined spaces.
- In synthesis, employ inert gas purging to prevent oxidation.
- Dispose via controlled flaring or supplier return programs.
- Emergency response: Evacuate and use foam extinguishers for fires.
Adhering to these ensures precision applications proceed without incident, safeguarding personnel and equipment.
8. Environmental Considerations and Sustainability
Propyne C3H4 gas contributes to sustainable practices by enabling cleaner welding with reduced emissions compared to fossil fuels. Its combustion produces primarily CO₂ and water, with low NOx under optimized conditions. In synthesis, it supports green chemistry principles through atom-efficient reactions.
Efforts to derive propyne from renewable sources, like biomass pyrolysis, are gaining traction, aligning with global carbon neutrality goals. Recycling cylinder residuals further minimizes waste, enhancing its eco-profile in precision-driven fields.
9. Cutting-Edge Research and Nuclear Integrations
In advanced research, propyne C3H4 gas interfaces with nuclear technologies, such as in plasma welding for reactor components, where its high-energy flame fuses exotic alloys. Isotopic forms aid in neutron activation analysis, tracing elemental distributions.
My work with rare gases shows propyne’s potential in fusion fuel mixtures, enhancing plasma stability. In organic synthesis for nuclear medicine, it builds radiolabeled compounds with precision, improving imaging agents.
10. Future Directions and Technological Horizons
The trajectory of propyne C3H4 gas points toward expanded precision in emerging technologies. Nanoscale welding for microelectronics and AI-optimized synthesis protocols promise breakthroughs. Integration with hydrogen economies could see propyne as a bridge fuel, driving hybrid systems.
As research evolves, propyne C3H4 gas will continue to refine gas welding and organic synthesis, embodying the precision that defines progress in chemical and nuclear domains.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )