Methyl Acetylene C3H4: A High-Purity Gas for Cutting-Edge Chemical Applications
BY STEVEN, Published August 17, 2025
Methyl acetylene, denoted as C3H4 and commonly referred to as propyne, emerges as a pivotal high-purity gas in the landscape of advanced chemical engineering and nuclear-related research. With decades of immersion in hydrocarbon gases and specialty formulations, I’ve observed how this alkyne’s distinctive triple bond drives innovations in synthesis, catalysis, and energy systems. Its high purity levels—often exceeding 99.5%—enable precise control in reactions where contaminants could derail outcomes, positioning methyl acetylene C3H4 as an essential tool for cutting-edge chemical applications that demand reliability and efficiency.
1. Molecular Composition and Intrinsic Characteristics
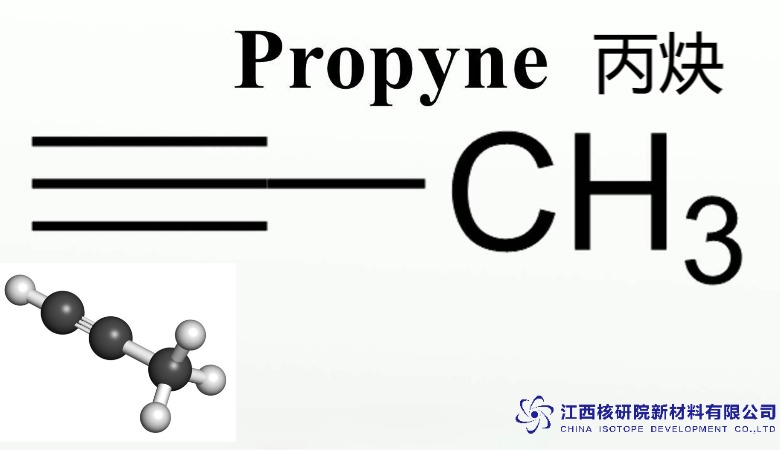
The essence of methyl acetylene C3H4 lies in its streamlined molecular architecture: a chain of three carbon atoms where the terminal carbons connect via a triple bond, with one end capped by a methyl group and the other by a hydrogen (CH3-C≡CH). This structure imparts a degree of polarity, enhancing its reactivity compared to non-polar hydrocarbons, while maintaining stability under controlled conditions. As a colorless, flammable gas with a subtle garlic-like scent, it transitions seamlessly between gaseous and liquid states, making it adaptable for various delivery systems in laboratory and industrial settings.
From a physicochemical standpoint, methyl acetylene C3H4 exhibits properties that optimize it for high-stakes applications. Its low molecular weight facilitates diffusion in gas mixtures, and the triple bond’s high energy content—around 115 kcal/mol—fuels exothermic reactions critical in organic transformations. In my extensive studies of carbon-hydrogen gases, I’ve noted how these traits align with demands in nuclear fuel processing, where purity directly influences isotopic separation efficiency.
For a concise reference, the following table outlines the fundamental properties of methyl acetylene C3H4, drawing from standardized measurements:
| Property | Value/Description |
|---|---|
| Molecular Formula | C3H4 |
| CAS Number | 74-99-7 |
| Molecular Weight | 40.063 g/mol |
| Appearance | Colorless gas |
| Odor | Faint, garlic-like |
| Density (gas at 0°C, 1 atm) | 1.787 kg/m³ |
| Melting Point | -101.5°C |
| Boiling Point | -23.2°C |
| Solubility in Water (20°C) | 3.64 g/L |
| Heat of Vaporization | 18.4 kJ/mol |
| Bond Dissociation Energy (C≡C) | ~490 kJ/mol |
These characteristics underscore its volatility, which necessitates specialized handling but also enables rapid integration into dynamic chemical processes.
2. Synthesis Pathways and Purification Techniques
Producing high-purity methyl acetylene C3H4 involves sophisticated methods honed over years of petrochemical advancements. The primary industrial route stems from the thermal cracking of propane or naphtha, yielding a crude mixture of alkynes and alkenes. Selective distillation then isolates methyl acetylene, often in conjunction with its isomer allene (propadiene), forming what’s known as MAPP gas—a blend valued for its stability.
In laboratory contexts, alternative syntheses include the reaction of acetylene with methyl halides in the presence of strong bases like sodium amide, or the dehydrohalogenation of 2-bromopropene. These approaches allow for tailored production, especially when incorporating isotopic labels such as deuterium for tracer studies in nuclear reactions. Purification escalates through cryogenic fractionation or adsorptive chromatography, achieving purities up to 99.999% by removing traces of hydrocarbons like ethylene or butadiene, which could compromise reaction selectivity.
My insights from specialty gas research reveal that advancements in membrane separation technologies are revolutionizing production, offering energy-efficient alternatives to traditional distillation. This not only boosts yield but also aligns with sustainable practices, reducing the carbon footprint associated with high-purity gas manufacturing.
3. Pivotal Roles in Organic Synthesis and Catalysis
Methyl acetylene C3H4 shines in organic synthesis, where its terminal alkyne functionality serves as a versatile synthon for constructing complex molecules. In cross-coupling reactions, such as the Sonogashira protocol, it couples with aryl halides under palladium catalysis to form conjugated systems essential for pharmaceuticals and electronic materials. This reactivity stems from the acidic hydrogen (pKa ~25), which deprotonates readily, facilitating nucleophilic additions.
In the realm of polymer chemistry, methyl acetylene contributes to the synthesis of polyacetylenes, conductive polymers with applications in flexible electronics. Its high-purity form ensures uniform chain lengths, enhancing material properties like conductivity and thermal stability. Furthermore, in asymmetric synthesis, chiral catalysts leverage methyl acetylene for enantioselective alkynylations, producing intermediates for agrochemicals and fine chemicals.
Key advantages in these applications include:
- Selective reactivity that minimizes side products.
- Compatibility with green solvents, promoting eco-friendly processes.
- Scalability from bench to industrial levels without purity loss.
- Integration with flow chemistry systems for continuous production.
These elements make methyl acetylene C3H4 indispensable for cutting-edge chemical applications, particularly in developing next-generation catalysts for hydrogen production via alkyne reforming.
4. Industrial Utilization in Welding and Energy Systems
Beyond synthesis, methyl acetylene C3H4 excels in industrial welding as a component of MAPP gas, delivering flame temperatures around 2,920°C—hotter than propane but safer than acetylene due to reduced acetylene content. This high-purity gas enables precise cutting of thick metals, with cleaner burns that produce less soot, ideal for aerospace and automotive fabrication.
In energy sectors, its combustion properties support studies in fuel cells and internal combustion engines, where blending with hydrogen enhances octane ratings and reduces emissions. From a nuclear perspective, methyl acetylene’s role in plasma torches for waste vitrification highlights its utility in high-temperature environments, aiding the containment of radioactive materials.
Industrial benefits encompass:
- Enhanced flame stability for intricate welding tasks.
- Lower explosion risks compared to pure acetylene.
- Versatility in gas mixtures for customized energy outputs.
- Contribution to biofuel research through alkyne-derived additives.
Such applications demonstrate how methyl acetylene C3H4 drives efficiency in energy-intensive processes, aligning with global pushes toward cleaner technologies.
5. Integration with Nuclear and Specialty Gas Technologies
Drawing from my expertise in nuclear elements, methyl acetylene C3H4 intersects with isotopic gases and heavy water systems in tracer applications. Deuterated variants (e.g., CD3-C≡CH) serve as probes in neutron scattering experiments, elucidating molecular dynamics in fusion reactors. Its compatibility with rare gases like argon in plasma-enhanced chemical vapor deposition (PECVD) facilitates thin-film coatings for nuclear fuel rods, improving corrosion resistance.
In mixed-gas formulations, combining methyl acetylene with fluorocarbons yields specialty blends for etching in semiconductor manufacturing, where high purity prevents defects in microelectronics. This synergy extends to environmental monitoring, using methyl acetylene as a calibration standard for volatile organic compound (VOC) analyzers in nuclear facilities.
6. Detailed Product Specifications and Operational Performance
High-purity methyl acetylene C3H4 is supplied in compressed cylinders, typically aluminum or steel, with volumes from 5 to 50 liters and pressures up to 15 bar. Purity grades range from technical (95%) to ultra-high (99.999%), with impurities like moisture limited to <5 ppm and other hydrocarbons <10 ppm.
Performance metrics include:
- Reactivity Index: High, with rapid addition to electrophiles at rates exceeding 10^3 M^-1 s^-1 under catalysis.
- Thermal Conductivity: 0.018 W/m·K at 25°C, suitable for heat transfer in reaction vessels.
- Combustion Enthalpy: -1,849 kJ/mol, providing substantial energy release.
- Shelf Life: Indefinite in sealed containers, though polymerization inhibitors like hydroquinone are added for stability.
In operational tests, methyl acetylene maintains integrity up to 200°C in inert atmospheres, with decomposition onset at 400°C yielding carbonaceous residues.
7. Essential Usage Guidelines and Safety Protocols
Utilizing methyl acetylene C3H4 requires adherence to stringent protocols to mitigate its flammability and potential for explosive decomposition. Always employ in well-ventilated areas with explosion-proof equipment, monitoring concentrations below 1,000 ppm to avoid asphyxiation.
Critical handling notes:
- Store cylinders vertically in shaded, fire-resistant cabinets below 50°C.
- Use regulators designed for acetylene-like gases to control flow precisely.
- Avoid contact with copper or silver, as acetylides may form and ignite.
- In emergencies, use dry chemical extinguishers; water can exacerbate reactions.
- Conduct leak checks with soap solutions, and ensure personnel wear SCBA in confined spaces.
Regular training and compliance with standards like NFPA 51B ensure safe deployment, maximizing the gas’s benefits in cutting-edge chemical applications.
8. Emerging Trends and Forward-Looking Innovations
The horizon for methyl acetylene C3H4 brims with potential, particularly in sustainable chemistry. Bio-based production from renewable feedstocks like biomass-derived propylene could supplant fossil routes, enhancing its eco-profile. In nanotechnology, alkyne-click chemistry using methyl acetylene enables precise assembly of nanostructures for drug delivery in nuclear medicine.
Advancements in computational modeling predict new catalytic cycles, optimizing methyl acetylene for carbon capture via alkyne-hydration pathways. Integration with AI-driven process control will further elevate its role in smart manufacturing, ensuring high-purity outputs in real-time.
As industries evolve, methyl acetylene C3H4’s adaptability will cement its status in cutting-edge chemical applications, fostering breakthroughs that blend efficiency with innovation.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )