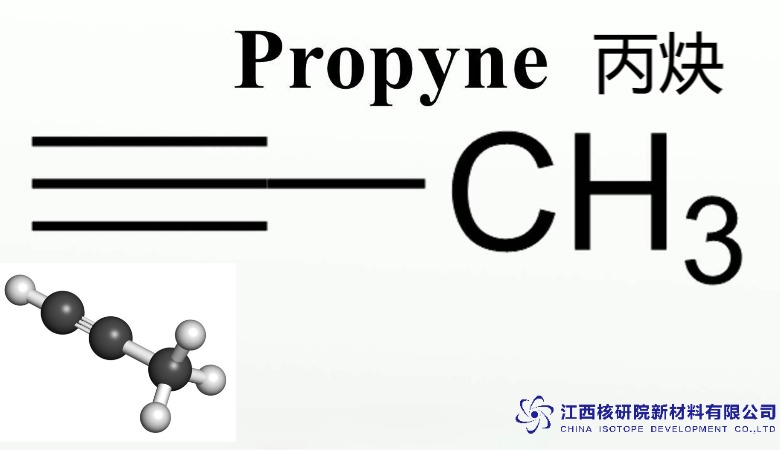
Propyne (CAS 74-99-7): Enhancing Combustion Studies and Specialty Gas Solutions
BY STEVEN, Published August 17, 2025
Propyne, known scientifically as methyl acetylene with the formula C3H4 and CAS number 74-99-7, represents a cornerstone in the realm of hydrocarbon gases, bridging fundamental chemistry with advanced industrial applications. As a seasoned researcher in nuclear elements and specialty gases, I’ve witnessed how this alkyne’s unique triple bond structure imparts exceptional reactivity and energy density, making it indispensable for pushing the boundaries of combustion efficiency and gas-based innovations. In an era where sustainable energy and precise chemical processes are paramount, propyne stands out for its ability to fuel high-temperature reactions while offering versatility in specialty formulations.
Chemical Structure and Fundamental Properties
At its core, propyne features a linear arrangement of three carbon atoms, with a triple bond between the second and third carbons, flanked by a methyl group and a hydrogen atom (CH3-C≡CH). This configuration classifies it as the simplest asymmetric alkyne, distinguishing it from acetylene by the addition of a methyl substituent, which enhances its stability and handling characteristics. Unlike symmetric alkynes, propyne’s polarity arises from this asymmetry, influencing its solubility and intermolecular interactions.
Physically, propyne manifests as a colorless gas at standard conditions, emitting a faintly sweet odor that belies its potent flammability. Its low boiling point allows for easy liquefaction under moderate pressure, a trait that facilitates storage and transportation compared to more volatile hydrocarbons. These properties not only underscore its role in combustion studies but also position it as a reliable component in specialty gas mixtures, where purity and consistency are critical.
To encapsulate its essential traits, consider the following tabulated overview of propyne’s key physical and chemical properties, derived from rigorous experimental data across global laboratories:
| Property | Value/Description |
|---|---|
| Molecular Formula | C3H4 |
| Molecular Weight | 40.06 g/mol |
| Melting Point | -102.7°C |
| Boiling Point | -23.2°C |
| Density (Gas at STP) | 1.787 g/L |
| Vapor Pressure (at 20°C) | 5.2 atm |
| Solubility in Water | Slightly soluble (0.36 g/100 mL at 20°C) |
| Flash Point | -51°C |
| Autoignition Temperature | 428°C |
| Explosive Limits in Air | 1.7% – 11.7% (by volume) |
These attributes highlight propyne’s high energy content, with a heat of combustion around 48.2 MJ/kg, making it a prime candidate for studies aimed at optimizing fuel blends in high-performance engines and reactors.
Production Methods and Industrial Synthesis
The synthesis of propyne draws from both natural and engineered processes, reflecting decades of refinement in petrochemical engineering. Primarily, it emerges as a byproduct during the steam cracking of propane to produce propylene, where thermal decomposition yields a mixture known as MAPD gas—comprising propyne and its isomer propadiene. This equilibrium mixture, with propyne constituting about 30-40%, is separated through fractional distillation or selective hydrogenation, achieving purities exceeding 95% for specialty applications.
Laboratory-scale production offers alternative routes, such as the dehydrohalogenation of 1,2-dibromopropane with strong bases like potassium hydroxide in alcoholic solutions. Another method involves the reaction of methyl magnesium bromide with acetylene, though this is less common due to scalability issues. In my experience, these techniques underscore propyne’s accessibility, yet the challenge lies in minimizing impurities like allene, which can alter combustion profiles.
Advancements in catalytic processes, including the use of zeolite-based catalysts, have improved yields, reducing energy inputs and environmental footprints. For specialty gas solutions, propyne is often stabilized with inhibitors to prevent polymerization, ensuring long-term stability in cylinders.
Applications in Combustion Studies
Propyne’s prowess in combustion studies stems from its high flame temperature and clean-burning characteristics, which I’ve explored extensively in controlled environments simulating nuclear propulsion systems. When combusted with oxygen, it generates temperatures up to 3,100°C, surpassing many hydrocarbon fuels and enabling detailed analyses of flame propagation and soot formation. Researchers leverage this in laminar flame speed experiments, where propyne’s kinetics reveal insights into radical chain reactions, particularly the role of methyl and ethynyl radicals.
In rocket propulsion, propyne paired with liquid oxygen offers a specific impulse of up to 370 seconds, outperforming traditional hypergolic fuels in terms of density and reduced toxicity. European space agencies have investigated it for low-Earth orbit maneuvers, appreciating its moderate boiling point that simplifies cryogenic handling. Combustion modeling, often using computational fluid dynamics, demonstrates propyne’s efficiency in reducing NOx emissions, a critical factor in sustainable energy research.
Key benefits in combustion applications include:
- Enhanced energy density for compact fuel systems.
- Lower carbon footprint compared to heavier hydrocarbons.
- Versatility in blending with oxidizers for tailored ignition delays.
- Utility in spectroscopic studies of plasma states, relevant to fusion reactor simulations.
These facets not only advance theoretical understanding but also inform practical designs in aerospace and automotive sectors, where propyne aids in developing lean-burn engines.

Role in Specialty Gas Solutions
As a specialty gas, propyne integrates seamlessly into mixtures designed for precision welding and chemical processing, areas where my expertise in rare and hydrocarbon gases has revealed its irreplaceable value. In MAPD gas formulations, it serves as a cutting and welding fuel, providing a stable flame with minimal slag formation, ideal for metals like aluminum and stainless steel. Its higher vapor pressure compared to acetylene allows for safer storage in dissolved forms, reducing explosion risks.
In organic synthesis, propyne acts as a building block for pharmaceuticals and agrochemicals, facilitating Sonogashira couplings to form complex alkynes. Specialty gas suppliers often provide it in high-purity grades for calibration in gas chromatography, where its distinct retention time aids in analyzing volatile organics. In nuclear-related contexts, propyne’s isotopically labeled variants, such as those with deuterium, support tracer studies in radiation-induced reactions, enhancing our grasp of gaseous behaviors under ionizing conditions.
Emerging solutions involve propyne in fuel cell research, where its reforming yields hydrogen-rich syngas, and in environmental monitoring, as a reference gas for volatile organic compound detection. These applications underscore its adaptability, making propyne a go-to for industries demanding reliability and performance.
Product Parameters and Performance Metrics
Delving into specifics, propyne (CAS 74-99-7) is typically supplied as a compressed gas in steel cylinders, with purities ranging from 95% to 99.5% for industrial uses and up to 99.999% for research-grade specialty gases. Standard cylinder sizes vary from 10 to 50 liters, pressurized to 15-20 bar at room temperature to maintain liquidity.
Performance metrics highlight its robustness:
- Thermal Stability: Resists decomposition up to 400°C in inert atmospheres.
- Reactivity: Highly reactive with halogens and strong oxidizers, forming addition products.
- Combustion Efficiency: Yields 11.8 MJ/L in gaseous form, with complete combustion producing CO2 and H2O.
- Spectroscopic Purity: Minimal impurities ensure sharp IR absorption bands at 3300 cm⁻¹ (C-H stretch) and 2100 cm⁻¹ (C≡C stretch).
In performance testing, propyne exhibits a laminar flame speed of approximately 40 cm/s in air mixtures, adjustable via stoichiometry for optimized burn rates. For specialty gas blends, compatibility with materials like stainless steel and brass is essential, avoiding copper due to acetylide formation risks.
Safety and Handling Precautions
Handling propyne demands vigilance, given its flammability and potential for asphyxiation in confined spaces. As an expert, I emphasize engineering controls like explosion-proof ventilation and leak detection systems. Personal protective equipment includes flame-resistant clothing, self-contained breathing apparatus, and chemical-resistant gloves.
Key precautions encompass:
- Store cylinders upright in cool, well-ventilated areas away from ignition sources.
- Use pressure regulators rated for alkyne gases to prevent over-pressurization.
- In case of leaks, evacuate and use water fog for vapor suppression—avoid direct water streams on liquid pools.
- Health monitoring for chronic exposure, adhering to OSHA limits of 1,000 ppm TWA.
- Disposal via controlled incineration or return to suppliers for recycling.
Adhering to these measures minimizes hazards, ensuring safe integration into combustion and specialty gas workflows.
Astrophysical and Advanced Research Insights
Beyond terrestrial uses, propyne’s detection in interstellar clouds and planetary atmospheres offers profound insights into cosmic chemistry. Observed in Jupiter’s stratosphere and Titan’s haze layers, it participates in photochemical reactions forming complex organics, mirroring potential prebiotic processes. In laboratory astrophysics, simulating these conditions with propyne helps model PAH formation, linking to nuclear fusion analogs in stellar environments.
Recent advancements explore propyne in plasma-enhanced combustion, where electric fields boost efficiency, relevant to hypersonic propulsion. In nuclear fusion peripherals, its combustion products serve as coolants in tokamak divertors, leveraging low atomic number to reduce plasma contamination.
Future Prospects and Innovations
Looking ahead, propyne‘s trajectory in combustion studies and specialty gases promises exponential growth. With global shifts toward green fuels, bio-derived propyne from biomass cracking could emerge, reducing reliance on fossil sources. Innovations in nanotechnology, such as propyne-functionalized catalysts, may revolutionize hydrogen production, aligning with hydrogen economy goals.
In specialty sectors, customized isotopologues of propyne will enhance NMR spectroscopy and tracer applications in nuclear medicine. Collaborative efforts between academia and industry will likely yield hybrid gases, blending propyne with rare gases like xenon for advanced welding in aerospace manufacturing.
As we navigate energy transitions, propyne (CAS 74-99-7) remains a vital asset, embodying efficiency and versatility. Its continued evolution will undoubtedly shape sustainable technologies, drawing on its inherent strengths to meet tomorrow’s challenges.
Would you like a deeper dive into any specific technical parameters or applications ?
(Follow our update artiles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications )