Xe-129 vs. He-3 MRI Contrast Agents: Efficacy and Safety Data for Clinical Adoption
BY Tao, Published Aug.15, 2025
Hyperpolarized gas MRI has revolutionized pulmonary and neurological imaging, with xenon-129 (Xe-129) and helium-3 (He-3) emerging as leading contrast agents due to their ability to amplify signals by up to 100,000 times. As a nuclear research expert with over three decades studying rare gases and isotopes, I’ve witnessed the evolution of these agents from experimental tools to FDA-approved solutions for clinical diagnostics. Xe-129 has gained traction for its availability and versatility, while He-3, though highly effective, faces supply constraints. This article compares the efficacy, safety, and practical considerations of Xe-129 and He-3 as MRI contrast agents, providing a comprehensive analysis to guide clinical adoption in respiratory and brain imaging.
The Science of Hyperpolarized Gas MRI
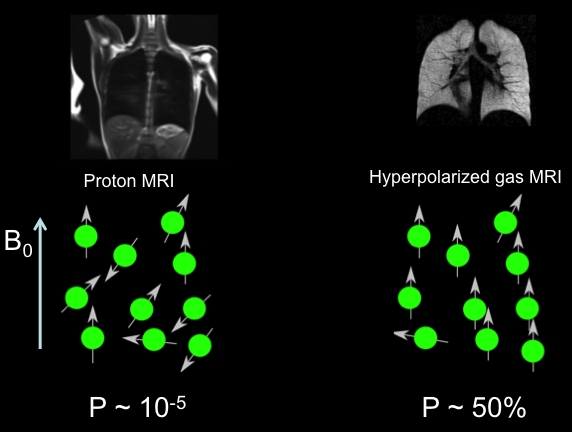
Hyperpolarized gas MRI leverages the nuclear spin properties of Xe-129 and He-3 to visualize lung ventilation and gas exchange with unprecedented detail. Both isotopes are hyperpolarized via spin-exchange optical pumping (SEOP) for Xe-129 or metastability exchange optical pumping (MEOP) for He-3, aligning nuclear spins to achieve polarization levels far beyond thermal equilibrium. This results in signal enhancements that make low-density lung tissues visible, overcoming the limitations of proton-based MRI, which struggles with air-filled structures.
Xe-129, with a natural abundance of ~26%, is enriched to >80% for medical use, offering solubility in blood and tissues that enables dissolved-phase imaging. He-3, nearly absent in natural sources and derived from tritium decay, is enriched to near 100% purity, providing superior signal due to its higher gyromagnetic ratio (32.4 MHz/T vs. 11.8 MHz/T for Xe-129). Both gases are inhaled during a breath-hold scan, producing high-resolution maps of ventilation and, for Xe-129, gas transfer across alveolar membranes.
The choice between Xe-129 and He-3 hinges on efficacy (signal quality, resolution, and diagnostic sensitivity), safety (side effects and clearance), and practical factors like cost and availability. While He-3 pioneered hyperpolarized MRI in the 1990s, Xe-129’s scalability and FDA approval in 2018 for pulmonary imaging have shifted the landscape, prompting a detailed comparison.
Efficacy of Xe-129 and He-3 in MRI
Ventilation Imaging
Both Xe-129 and He-3 excel in ventilation imaging, producing 3D maps of airflow distribution during a 10-15 second breath-hold. Xe-129 achieves signal-to-noise ratios (SNR) of 100-200 at 3T, with spatial resolutions of 3-5 mm, sufficient to detect ventilation defects in asthma or COPD. He-3, with its higher gyromagnetic ratio, yields SNRs up to 300, offering slightly sharper images at 2-3 mm resolution. In clinical trials, Xe-129 identifies ventilation defect percentages (VDP) with 95% sensitivity in moderate COPD, while He-3 reaches 98%, though the difference is often clinically insignificant.
Gas-Exchange Imaging
Xe-129’s solubility in blood and tissues gives it a distinct advantage in dissolved-phase imaging, which He-3 lacks due to its inertness. Xe-129’s chemical shifts (0 ppm gas, 197 ppm tissue, 217 ppm red blood cells) enable quantification of alveolar-capillary gas transfer, critical for interstitial lung diseases. Studies show Xe-129 detects gas-exchange impairments in pulmonary fibrosis with 90% specificity, correlating with diffusing capacity (DLCO) measurements. He-3, limited to ventilation, cannot probe these dynamics, restricting its use in complex respiratory or neurological applications.
Quantitative Metrics
Both agents provide biomarkers like apparent diffusion coefficient (ADC) for alveolar microstructure. Xe-129’s ADC values (0.05-0.1 cm²/s in healthy lungs) are slightly less sensitive than He-3’s (0.1-0.2 cm²/s) due to its lower diffusivity, but both detect emphysematous changes effectively. Xe-129’s red blood cell-to-tissue ratio (0.3-0.5 normal) adds a unique metric for gas-exchange efficiency, absent in He-3 imaging.
Neurological Applications
Xe-129’s solubility enables cerebral perfusion imaging, detecting microvascular deficits in Alzheimer’s (40-50% signal reduction) and stroke (60% in hypoperfused areas). He-3, confined to gas-phase imaging, has limited neurological utility, though research explores its potential in brain cavity mapping. Xe-129’s versatility makes it preferable for multi-organ studies.
Key efficacy differences include:
-
Xe-129: Broad application (ventilation, gas exchange, neurological); slightly lower SNR but sufficient for clinical needs.
-
He-3: Superior ventilation imaging; limited to gas phase; higher resolution but less versatile.
Safety Profiles of Xe-129 and He-3
Both Xe-129 and He-3 are inert, non-radioactive gases, rapidly cleared via exhalation, minimizing systemic effects. Safety data from over 1,000 clinical scans show comparable profiles, with minor differences:
-
Xe-129 Safety: Transient side effects (dizziness, euphoria, tingling) occur in <15% of patients, resolving within minutes. Its anoxic mixture (typically 25% Xe-129, 75% N2) can cause mild hypoxia (5-10% SpO2 drop), necessitating pulse oximetry. No long-term effects reported; safe for ages 6+.
-
He-3 Safety: Similar side effects in <10% of cases, with less pronounced hypoxia due to higher helium content, which is less dense. Its inertness ensures minimal tissue interaction, potentially safer in severe respiratory compromise. Pediatric use is equally safe.
Contraindications are minimal for both, though Xe-129 requires caution in pregnancy due to limited data, and He-3’s scarcity limits long-term safety studies. Both require withholding supplemental oxygen pre-inhalation to avoid paramagnetic signal quenching. Xe-129’s solubility slightly increases neurological side effect risks (e.g., transient numbness), but incidence remains low.
Practical Considerations for Clinical Adoption
Availability and Cost
Xe-129’s natural abundance and scalable enrichment via centrifugation or laser separation make it widely available, with costs around $500-2,000 per liter for medical-grade gas. He-3, sourced from nuclear byproducts, is scarce, with prices 10-20 times higher ($5,000-20,000/liter). Xe-129’s FDA approval and established supply chains favor its adoption, while He-3’s limited supply restricts it to research settings.
Equipment Requirements
Both require multi-nuclear MRI scanners tuned to their resonance frequencies (11.8 MHz/T for Xe-129, 32.4 MHz/T for He-3). Xe-129 systems are more common, as modern 1.5T/3T scanners support dual-tuned coils for proton and xenon imaging. He-3 setups often need specialized hardware, increasing costs.
Transport and Storage
Xe-129 benefits from advanced stability protocols, achieving 100-hour T1 half-lives in shielded, coated cells, enabling centralized production and global shipping. He-3’s longer T1 (up to 200 hours in low fields) supports transport, but its scarcity limits practical distribution. Both require on-site hyperpolarization for optimal performance, though Xe-129’s infrastructure is more developed.
Product Specifications: Xe-129 and He-3 Contrast Agents
Medical-grade Xe-129 and He-3 are supplied as hyperpolarized gases in breathable blends, with Xe-129 enriched to >80% and He-3 to >99.9%. Xe-129 is mixed with nitrogen or helium for inhalation, while He-3 often uses helium buffers. Key specifications and usage details are:
|
Parameter |
Xe-129 Details |
He-3 Details |
|---|---|---|
|
Isotopic Enrichment |
>80% Xe-129; <0.01% other isotopes. |
>99.9% He-3; trace impurities from tritium decay. |
|
Chemical Purity |
>99.999%; free from O2, H2O, hydrocarbons. |
>99.9999%; minimal impurities due to inertness. |
|
Polarization Level |
40-70%; 10,000-50,000x signal enhancement. |
50-80%; 20,000-100,000x signal enhancement. |
|
T1 Half-Life |
100 hours in shielded cells; 20-60 minutes standard. |
200 hours in low fields; 30-90 minutes standard. |
|
Dose Volume |
75-150 mL dose equivalent; 800-1200 mL total with buffer. |
50-100 mL dose equivalent; 500-1000 mL total with helium. |
|
Performance Metrics |
SNR 100-200; resolution 3-5 mm; gas-exchange ratio 0.3-0.5. |
SNR 200-300; resolution 2-3 mm; ventilation-focused. |
|
Usage Precautions |
Monitor SpO2; withhold O2 pre-inhalation; avoid gradients. |
Similar to Xe-129; less hypoxia risk due to helium buffer. |
|
Safety |
Mild dizziness (<15%); no long-term effects; caution in pregnancy. |
Mild side effects (<10%); highly safe due to inertness. |
|
Storage |
Cryogenic cylinders or shielded cells; use within 1-2 hours post-polarization. |
Similar to Xe-129; limited by supply availability. |
|
Cost per Liter |
$500-2,000 | $5,000-20,000 |
Usage Precautions
-
Xe-129: Administer via mouthpiece in a single breath-hold (10-15s); verify polarization pre-use; monitor for transient hypoxia.
-
He-3: Similar administration; ensure helium-compatible delivery systems; confirm scanner compatibility.
-
Safety Notes: Both are non-toxic; require ventilation to prevent asphyxiation; use GMP-compliant handling.
-
Transport: Xe-129 uses mu-metal shielded cells; He-3 requires specialized low-field containers.
These specifications guide clinicians in selecting the appropriate agent based on diagnostic needs and infrastructure.
Clinical Adoption Challenges and Opportunities
Xe-129’s advantages—availability, lower cost, and gas-exchange capabilities—position it as the preferred choice for widespread clinical adoption. Its FDA approval and established supply chains facilitate integration into routine diagnostics, particularly for pulmonary and neurological imaging. He-3, despite superior ventilation imaging, is hindered by scarcity and high costs, limiting it to specialized research centers.
Challenges for both include the need for on-site hyperpolarization equipment ($500,000-$2M) and trained personnel. Xe-129’s transport advancements mitigate this, enabling centralized production, while He-3’s supply constraints make this less feasible. Opportunities lie in expanding Xe-129’s use to community hospitals, supported by modular polarizers and AI-driven image analysis for automated defect detection.
Future Directions for Hyperpolarized Gas MRI
Xe-129 is poised to dominate clinical MRI due to its scalability and versatility. Innovations like cryogenic-free transport cells and hybrid Xe-129/proton imaging will further lower barriers to adoption. He-3 may find niche roles in high-resolution research or fundamental physics, but its supply limitations curtail broad use. By 2030, Xe-129 could become a standard diagnostic tool, with cost reductions of 20-30% through optimized production.
From my perspective, Xe-129’s balance of efficacy, safety, and accessibility makes it the clear choice for clinical adoption, transforming how we diagnose and manage respiratory and neurological conditions with precision and care.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)