Hyperpolarized Xe-129 Gas: Technical Breakthroughs in Neurological Disorder Diagnosis
BY Tao, Published Aug.15, 2025
The integration of hyperpolarized Xe-129 gas into magnetic resonance imaging has ushered in a new era for diagnosing neurological disorders, offering unprecedented sensitivity to brain perfusion, gas exchange, and tissue integrity. This noble gas isotope, when hyperpolarized, amplifies MRI signals by up to 100,000 times, enabling detailed visualization of cerebral blood flow and functional responses that traditional proton MRI often overlooks. As a nuclear research expert with decades exploring rare gases and isotopes, I’ve observed how hyperpolarized Xe-129 gas is pivotal in detecting early signs of conditions like Alzheimer’s disease and stroke, where subtle perfusion deficits can signal impending neurodegeneration. This article examines the technical innovations, clinical applications, and practical considerations of hyperpolarized Xe-129 gas in neurological diagnostics, emphasizing its role in enhancing precision medicine for brain health.
1. The Science of Hyperpolarized Xe-129 Gas in Brain Imaging
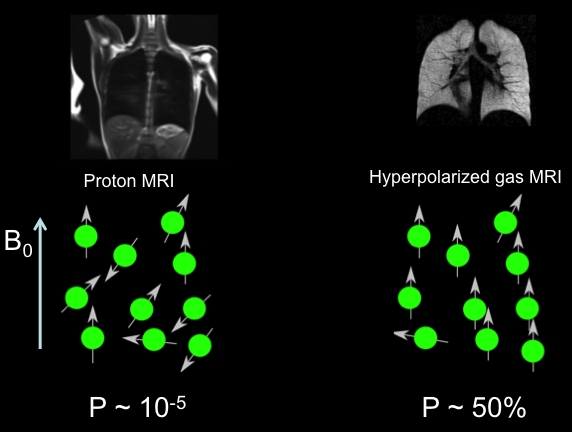
Hyperpolarized Xe-129 gas leverages the unique nuclear spin properties of xenon-129 to achieve extraordinary signal enhancement in MRI. Unlike conventional MRI, which relies on proton signals from water, hyperpolarized Xe-129 introduces an exogenous contrast agent that dissolves in blood and tissues, providing direct insights into perfusion dynamics. The process begins with spin-exchange optical pumping, where Xe-129 gas is exposed to circularly polarized laser light alongside rubidium vapor, aligning nuclear spins to levels far exceeding thermal equilibrium—typically 30-50% polarization for clinical volumes.
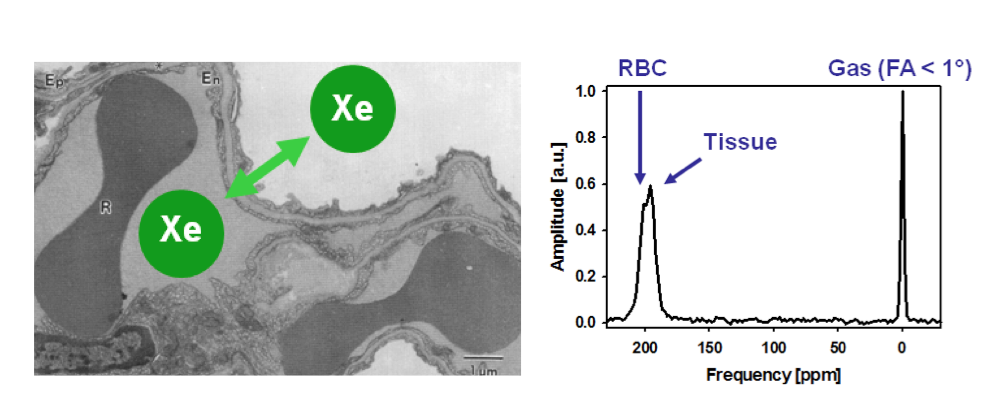
Once inhaled, hyperpolarized Xe-129 diffuses across the alveolar-capillary barrier, enters the bloodstream, and reaches the brain within seconds. Its solubility allows it to partition into different compartments: gas phase at 0 ppm, tissue/plasma at approximately 197 ppm, and red blood cells at 217 ppm. These distinct chemical shifts enable compartment-specific imaging, revealing perfusion patterns and blood-brain barrier integrity. Recent technical breakthroughs include the development of 3D gradient-echo sequences with non-Cartesian k-space sampling, which improve spatial resolution to 3-5 mm isotropic voxels, and chemical shift saturation recovery methods that quantify gas transfer kinetics with high temporal precision.
Historically, brain imaging with hyperpolarized gases started with helium-3, but its scarcity prompted a shift to Xe-129 by the early 2010s. Advancements in polarizer technology, such as continuous-flow systems yielding up to 1 liter of hyperpolarized gas at 50% polarization, have made Xe-129 viable for routine use. In neurological contexts, these innovations allow mapping of cerebral blood flow with sensitivities rivaling positron emission tomography, but without radiation exposure. For instance, time-of-flight techniques track Xe-129 transit through cerebral vasculature, detecting hemodynamic responses with signal-to-noise ratios exceeding 10 in healthy brains.
Key technical parameters influencing performance include:
- Polarization Efficiency: Achieved levels of 40-60% enhance signal by 10,000-50,000x, critical for low-concentration brain uptake (1-2% of inhaled dose).
- Relaxation Times: T1 in cerebral blood ranges from 3-8 seconds, while T2* varies by compartment, necessitating optimized pulse sequences to minimize decay.
- Field Strength Compatibility: Effective at 1.5T and 3T, with higher fields improving spectral resolution for multi-compartment analysis.
These elements combine to make hyperpolarized Xe-129 a breakthrough tool for probing neurological pathophysiology at the molecular level.
2. Breakthroughs in Imaging Techniques
Recent years have seen remarkable progress in hyperpolarized Xe-129 MRI protocols tailored for the brain. One major advance is the implementation of iterative decomposition with echo asymmetry and least-squares estimation (IDEAL) combined with spiral readouts, which separates signals from brain tissue, red blood cells, and plasma with sub-second temporal resolution. This technique has achieved signal-to-noise ratios of 30+ in rat models, enabling dynamic monitoring of gas wash-in and wash-out.
Another innovation is 3D chemical shift imaging, which provides volumetric maps of Xe-129 distribution, correlating with gray matter density in human subjects. Studies from 2023-2025 have refined these methods using multi-channel phased-array coils, boosting sensitivity by 2-3 fold and reducing voxel sizes to clinically relevant levels. For perfusion quantification, models incorporating diffusion coefficients and bidirectional gas transfer have been developed, accounting for Xe-129’s retrograde flow from brain tissue back to blood.
In functional imaging, hyperpolarized Xe-129 detects hemodynamic responses to stimuli, akin to blood oxygenation level-dependent fMRI but with superior specificity to perfusion changes. Time-of-flight sequences, for example, capture activation in motor and visual cortices during tasks, with perfusion maps showing 20-30% signal increases in responsive regions. These breakthroughs address previous limitations in spatial coverage, paving the way for whole-brain assessments in neurological patients.
3. Clinical Applications in Neurological Disorders
Hyperpolarized Xe-129 gas excels in diagnosing neurological disorders by highlighting perfusion deficits, barrier impairments, and functional alterations. Its non-invasive nature makes it ideal for serial monitoring, particularly in progressive conditions.
In Alzheimer’s disease, hyperpolarized Xe-129 reveals reduced gray matter uptake—up to 43% lower than in controls—due to microvascular dysfunction. Washout kinetics serve as a biomarker, with half-lives prolonged to 42-43 seconds in affected tissues versus 16-20 seconds in healthy ones, potentially detecting amyloid-related changes years before symptoms. Clinical trials since 2023 have integrated Xe-129 with proton MRI to map beta-amyloid deposition indirectly through altered gas exchange.
For stroke, Xe-129 imaging identifies hypoperfused regions with 60% signal reductions, correlating with infarct volumes and collateral flow. In acute cases, it quantifies mean transit times, aiding thrombolysis decisions, while in chronic stroke, it monitors recovery by tracking perfusion restoration. Recent studies (2024-2025) have shown its superiority over arterial spin labeling in detecting subtle penumbral changes.
Other applications include:
- Parkinson’s Disease: Assesses substantia nigra perfusion deficits, linking to dopaminergic loss.
- Multiple Sclerosis: Measures blood-brain barrier permeability, identifying active lesions with elevated tissue uptake.
- Traumatic Brain Injury: Tracks post-injury hyperemia or hypoperfusion, guiding rehabilitation.
- Epilepsy: Maps ictal hyperperfusion during seizures for surgical planning.
These uses stem from Xe-129’s ability to probe microvascular integrity, offering quantitative metrics like apparent diffusion coefficients (0.05-0.1 cm²/s in normal brain) and perfusion rates (50-80 mL/100g/min in gray matter).
4. Product Specifications and Performance
Hyperpolarized Xe-129 gas for neurological imaging is produced via on-site polarizers, enriched to >80% isotopic purity, and blended with nitrogen or helium for inhalation. A typical dose is 75-150 mL dose equivalent, calculated as volume × enrichment × polarization, delivered in 800-1200 mL bags.
Performance metrics highlight its efficacy: at 3T, brain tissue SNR reaches 20-40, enabling 3D maps with 4 mm resolution. In healthy subjects, gray matter perfusion averages 60 mL/100g/min, while deviations in disorders like Alzheimer’s drop to 40-50 mL/100g/min. Reproducibility is within 5-10% for repeated scans, with T1 relaxation supporting 10-20 second breath-holds.
Detailed specifications include:
| Aspect | Details |
|---|---|
| Composition | Hyperpolarized Xe-129 (>80% enrichment) with N2/He buffer; purity >99.99%. |
| Dose Equivalent | 75-150 mL; adjusted for brain uptake (1-2% of inhaled). |
| Polarization | 40-60%; signal boost 10,000-100,000x; T1 3-8s in blood. |
| Administration | Single or multiple breath-holds (10-20s); via mouthpiece in supine position. |
| Imaging Parameters | 1.5T/3T; 3D GRE/CSI sequences; resolution 3-5 mm; SNR 20-40. |
| Performance Metrics | Perfusion: 50-80 mL/100g/min gray matter; ADC: 0.05-0.1 cm²/s; HDR detection >20% signal change. |
| Precautions | Monitor SpO2 and ECG; withhold O2 to avoid quenching; transient hypoxia risk in compromised patients. |
| Side Effects | Mild dizziness/euphoria (<10%); resolves in minutes; no long-term effects. |
| Contraindications | Severe hypoxia, pregnancy (limited data); caution in acute stroke. |
| Storage/Preparation | Fresh hyperpolarization; use within 30-60 minutes; requires GMP-compliant polarizer. |
These parameters ensure safe, effective deployment in neurological settings.
5. Administration and Safety Considerations
Administering hyperpolarized Xe-129 involves a controlled inhalation protocol: patients inhale the gas mixture during a breath-hold, synchronized with MRI acquisition. Multi-nuclear scanners tuned to 11.8 MHz (at 1T) for Xe-129 are essential, with dual-tuned coils for co-registered proton images.
Safety is paramount; the inert gas clears rapidly via exhalation, with no residual effects. Precautions include continuous monitoring for transient desaturation (5-10% drop), especially in neurological patients with compromised autoregulation. Doses are titrated based on lung capacity, and protocols avoid high oxygen mixes to prevent paramagnetic signal loss. In over 500 reported scans, adverse events are rare and mild.
6. Challenges and Emerging Innovations
Despite breakthroughs, challenges persist: low brain uptake demands high polarization to combat SNR limitations, while breath-hold variability affects reproducibility in impaired patients. Equipment costs and the need for specialized coils hinder widespread adoption.
Innovations addressing these include portable polarizers for clinic integration and AI-driven analysis for automated perfusion mapping. Low-field systems (0.5T) show promise for cost-effective imaging, and combined Xe-129/proton sequences enhance anatomical correlation. Ongoing trials (2024-2025) explore Xe-129 with molecular probes for targeted Alzheimer’s imaging.
7. Advancing Neurological Diagnostics with Hyperpolarized Xe-129
Hyperpolarized Xe-129 gas represents a transformative breakthrough in neurological disorder diagnosis, illuminating perfusion and functional deficits with unmatched detail. By enabling early detection in Alzheimer’s and precise mapping in stroke, it promises to shift paradigms toward proactive interventions. As technologies evolve and accessibility improves, hyperpolarized Xe-129 will likely become integral to brain health assessments, drawing on nuclear science to unlock deeper understandings of neurological resilience.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)