Medical-Grade Xe-129 Isotope: Production Purity >99.99% for Clinical Lung Imaging
BY Tao, Published Aug.15, 2025
In the evolving landscape of diagnostic imaging, medical-grade Xe-129 isotope stands out as a cornerstone for non-invasive pulmonary assessments. With its exceptional nuclear properties and compatibility with magnetic resonance imaging (MRI), this stable xenon isotope enables hyperpolarized gas techniques that reveal intricate details of lung ventilation and gas exchange. As a nuclear research expert with over three decades immersed in rare gases and isotopes, I’ve seen how high-purity Xe-129 transforms clinical practices, offering signal enhancements that make subtle respiratory pathologies visible. This article delves into the production, purity standards, applications, and practical aspects of medical-grade Xe-129, highlighting its pivotal role in advancing clinical lung imaging.
1. The Role of Xe-129 in Advanced Medical Imaging
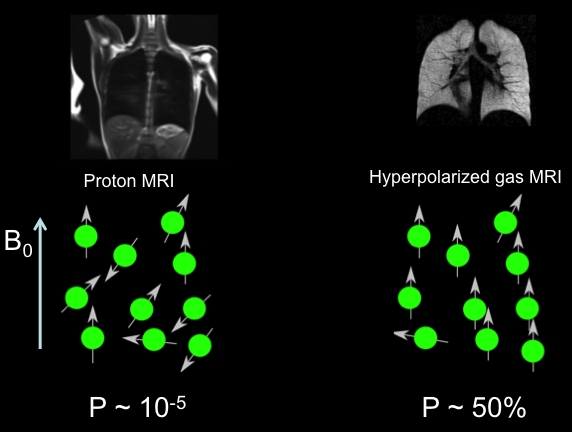
Xenon-129, a non-radioactive isotope of the noble gas xenon, possesses a spin-1/2 nucleus ideal for MRI applications. Unlike conventional proton-based MRI, which struggles with the low-density environment of the lungs, Xe-129 can be hyperpolarized to amplify signals dramatically—up to 100,000 times stronger than thermal equilibrium states. This hyperpolarization process aligns nuclear spins using laser optical pumping, allowing inhaled Xe-129 to map regional lung function with high spatial resolution.
The isotope’s natural abundance is approximately 26% in atmospheric xenon, but for medical use, it must be enriched to ultra-high isotopic purity levels exceeding 99.99%. This purity ensures minimal interference from other xenon isotopes, such as Xe-131 or Xe-132, which could dilute the signal or introduce artifacts in imaging. In clinical settings, medical-grade Xe-129 is blended with inert gases like helium and nitrogen to facilitate safe inhalation and optimal polarization. Its solubility in blood and tissues further extends its utility beyond ventilation imaging to probing gas diffusion across alveolar membranes, making it indispensable for diagnosing conditions like chronic obstructive pulmonary disease (COPD), asthma, and interstitial lung diseases.
From my extensive experience with rare gas isotopes, the shift toward Xe-129 represents a significant leap over earlier agents like helium-3, which, despite excellent imaging properties, suffers from supply shortages due to its derivation from nuclear byproducts. Xe-129’s abundance and enrichability position it as a sustainable, cost-effective alternative, with ongoing refinements in production techniques pushing purity boundaries to meet stringent medical standards.
2. Production Processes for Medical-Grade Xe-129
Producing medical-grade Xe-129 involves a multi-step process that begins with the extraction and enrichment of the isotope from natural xenon sources. Xenon is first isolated from air through fractional distillation, where it constitutes about 0.087 parts per million. The crude xenon mixture then undergoes isotopic separation, typically via centrifugation or electromagnetic methods, to concentrate Xe-129.
Centrifugal enrichment is the most common industrial approach, leveraging differences in atomic mass to separate isotopes in high-speed rotors. This method achieves enrichment levels up to 99.99% or higher, critical for medical applications where even trace impurities can compromise hyperpolarization efficiency. Post-enrichment, the Xe-129 is purified through cryogenic distillation and getter-based systems to remove chemical contaminants like oxygen, water vapor, or hydrocarbons, ensuring the final product meets pharmacopeial standards for sterility and safety.
Once enriched, the Xe-129 is prepared for hyperpolarization using spin-exchange optical pumping (SEOP). In this technique, the gas is mixed with an alkali metal vapor, such as rubidium, and exposed to circularly polarized laser light at 795 nm. The laser polarizes the rubidium electrons, which transfer spin alignment to Xe-129 nuclei through collisions. Modern systems, like automated hyperpolarizers, operate in continuous-flow or batch modes, producing hyperpolarized Xe-129 at rates sufficient for clinical throughput—up to 1 liter per session with polarization levels of 30-50%.
Key stages in the production workflow include:
- Extraction and Initial Purification: Air separation yields xenon, followed by removal of krypton and other volatiles.
- Isotopic Enrichment: Gas centrifuge cascades boost Xe-129 concentration to >99.99%.
- Quality Control: Spectroscopic analysis verifies purity, with mass spectrometry confirming isotopic composition.
- Hyperpolarization: On-site SEOP integrates the enriched gas into medical blends for immediate use.
These processes demand specialized facilities compliant with Good Manufacturing Practices (GMP), ensuring traceability from raw material to patient administration.
3. Achieving Ultra-High Purity in Xe-129 Isotope
Purity is paramount in medical-grade Xe-129, where isotopic enrichment above 99.99% minimizes signal loss and maximizes imaging contrast. Impurities, even at parts-per-billion levels, can quench polarization by promoting spin relaxation or chemical reactions. For instance, paramagnetic contaminants like oxygen accelerate T1 decay, reducing the usable lifespan of hyperpolarized Xe-129 from minutes to seconds.
To achieve such purity, producers employ advanced techniques like laser isotope separation or multi-stage centrifugation, often combined with cryogenic traps that selectively condense unwanted isotopes. Chemical purity is further enhanced through activated carbon adsorption and high-vacuum distillation, targeting >99.999% overall gas quality. In my career studying rare gas purification, I’ve noted that these methods not only boost efficiency but also reduce costs by recycling off-spec material.
Regulatory bodies, such as the FDA, mandate rigorous testing for medical-grade Xe-129, including assays for isotopic ratio, residual solvents, and microbial contamination. A typical certificate of analysis might report Xe-129 content at 99.995%, with total impurities below 10 ppm. This ultra-high purity enables consistent hyperpolarization fractions, directly translating to superior MRI performance in clinical lung imaging.
4. Applications in Clinical Lung Imaging
Medical-grade Xe-129 isotope revolutionizes clinical lung imaging by providing functional insights unattainable with standard radiography or CT scans. In hyperpolarized Xe-129 MRI, patients inhale the gas during a brief breath-hold, allowing scanners to capture 3D ventilation maps that highlight airflow distribution across lung lobes. This is particularly valuable for detecting early-stage ventilation defects in COPD, where traditional spirometry might overlook regional impairments.
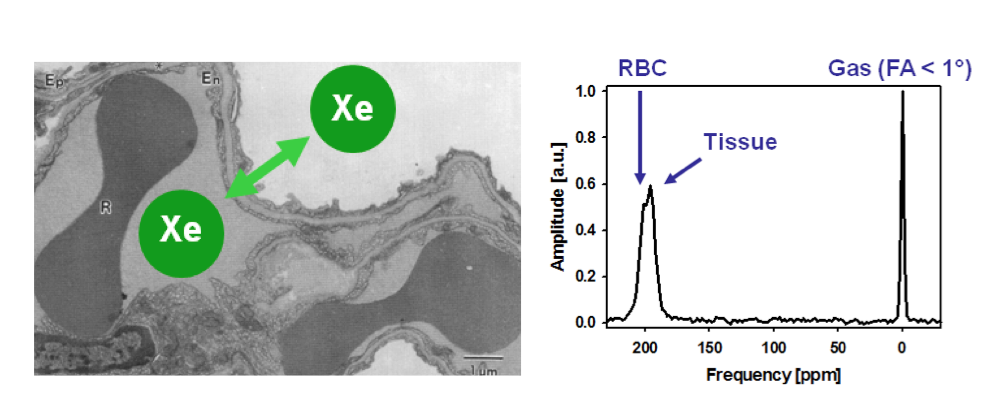
Beyond ventilation, Xe-129’s tissue solubility facilitates dissolved-phase imaging, quantifying gas transfer from alveoli to blood. Chemical shift differences—0 ppm for gas phase, 197 ppm for tissue, and 217 ppm for red blood cells—enable compartment-specific analysis, aiding in the diagnosis of pulmonary fibrosis or embolism. In pediatric cases, its non-ionizing nature makes it safer than nuclear medicine alternatives, with applications extending to cystic fibrosis monitoring and post-transplant evaluations.
Clinical studies demonstrate Xe-129 MRI’s sensitivity: ventilation defect percentages (VDP) correlate strongly with disease severity, often revealing abnormalities in 20-30% of asymptomatic smokers. For asthma management, it maps heterogeneous airway responses to bronchodilators, guiding personalized therapies. In interstitial diseases, apparent diffusion coefficients (ADC) from Xe-129 diffusion-weighted imaging assess alveolar enlargement, with values exceeding 0.1 cm²/s indicating emphysema progression.
The versatility of Xe-129 extends to emerging uses like assessing radiation-induced lung injury or evaluating novel therapeutics in clinical trials. Its integration with proton MRI provides hybrid anatomical-functional views, enhancing diagnostic accuracy for complex respiratory conditions.
5. Product Specifications and Performance Metrics
Medical-grade Xe-129 is typically supplied as an enriched gas blend for on-site hyperpolarization, with specifications tailored to clinical demands. The core product features isotopic purity >99.99%, ensuring optimal signal-to-noise ratios in MRI. A standard dose involves 75-100 mL of hyperpolarized Xe-129 (dose equivalent), diluted in 1 liter of buffer gas for safe inhalation.
Performance is gauged by polarization efficiency, often reaching 40-60% in commercial systems, yielding signal enhancements of 10,000-50,000x. In healthy lungs, ventilation coverage exceeds 95%, with ADC values of 0.05-0.08 cm²/s and gas-exchange ratios of 0.3-0.5. Diseased states show deviations, such as VDP >15% in moderate COPD, enabling quantitative tracking of therapeutic outcomes.
For comprehensive details, consider the following table summarizing key parameters:
| Aspect | Details |
|---|---|
| Isotopic Purity | >99.99% Xe-129 enrichment; <0.01% other xenon isotopes. |
| Chemical Purity | >99.999% overall; impurities <10 ppm (e.g., O2, H2O, hydrocarbons). |
| Dose Equivalent | 75-100 mL hyperpolarized Xe-129; total inhalation volume 800-1,200 mL. |
| Polarization Level | 40-60% achievable; T1 relaxation time 20-30 minutes in storage. |
| Imaging Compatibility | 1.5T or 3T MRI scanners; multi-nuclear coils required. |
| Performance Metrics | SNR >100 for ventilation; resolution 3-5 mm; reproducibility <5% variation. |
| Storage Conditions | Cryogenic or high-pressure cylinders; hyperpolarized state on-demand. |
| Shelf Life | Enriched gas: indefinite; hyperpolarized: use within 1 hour. |
These specifications ensure reliable performance in diverse clinical environments.
6. Administration and Safety Considerations
Administering medical-grade Xe-129 requires specialized protocols to maximize efficacy while minimizing risks. The process begins with hyperpolarization on-site, followed by transfer to a breathable bag. Patients inhale the mixture in a single breath, holding for 10-15 seconds during MRI acquisition. Pre-inhalation, supplemental oxygen is withheld to avoid paramagnetic quenching, and vital signs are monitored via pulse oximetry.
Safety is inherent to Xe-129’s inert nature, with rapid exhalation clearing the gas within seconds. Transient side effects, affecting <15% of users, include mild dizziness, euphoria, or tingling due to brief hypoxia. Contraindications are minimal, but caution is advised in severe respiratory compromise or pregnancy, pending further data. Usage precautions emphasize GMP-compliant handling to prevent contamination and regular equipment calibration for consistent polarization.
In practice, doses are titrated based on patient lung volume, with pediatric adjustments for ages 6 and up. Post-scan, patients resume normal activities immediately, underscoring Xe-129’s patient-friendly profile.
7. Challenges and Innovations in Xe-129 Technology
Despite its advantages, challenges persist in scaling medical-grade Xe-129 production. Enrichment costs remain high, though economies of scale are emerging with increased demand. Hyperpolarization’s transient nature necessitates proximity to imaging suites, limiting accessibility in remote areas. Additionally, scanner compatibility requires upgrades for multi-nuclear capabilities.
Innovations are addressing these hurdles: portable hyperpolarizers enable decentralized use, while AI-enhanced image analysis automates defect quantification. Research into higher enrichment techniques, potentially reaching 99.999%, promises even greater signal fidelity. Hybrid modalities combining Xe-129 with other isotopes or modalities could expand applications to brain or cardiac imaging.
8. Advancing Respiratory Care with Xe-129
Medical-grade Xe-129 isotope, with its unparalleled purity and imaging prowess, is redefining clinical lung imaging. By providing detailed, functional views of pulmonary health, it empowers clinicians to intervene earlier and more precisely in respiratory diseases. As production technologies mature and adoption grows, Xe-129 stands to become a staple in global healthcare, bridging the gap between diagnosis and personalized treatment. From my vantage point in nuclear research, this isotope exemplifies how meticulous science can illuminate the unseen, fostering better outcomes for millions affected by lung disorders.
Would you like a deeper dive into any specific technical parameters or applications?
(Follow up our update articles on www.asiaisotopeintl.com or send your comments to tao.hu@asiaisotope.com for further communications)