The Science Behind Nitrogen-15 Enrichment: Methods and Cutting-Edge Uses
BY Tao, Published Aug 13, 2025
1. The Importance of Nitrogen-15 Enrichment
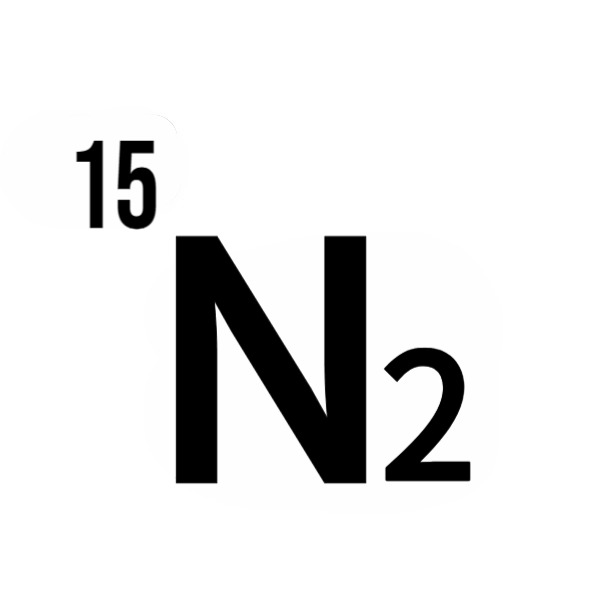
Nitrogen-15 (¹⁵N), a stable isotope of nitrogen with an extra neutron compared to the abundant Nitrogen-14 (¹⁴N), holds a pivotal role in advancing scientific and industrial applications. With a natural abundance of just 0.37%, ¹⁵N requires enrichment to unlock its potential in fields like agriculture, medicine, and materials science. As a nuclear research expert with decades of experience in isotopic technologies, I’ve seen how ¹⁵N’s unique nuclear properties—particularly its spin-1/2 nucleus—enable precise tracing and high-resolution spectroscopy, unlike the quadrupolar ¹⁴N. Enrichment transforms this scarce isotope into a powerful tool, amplifying its detectability in nuclear magnetic resonance (NMR) and mass spectrometry. This article explores the science behind ¹⁵N enrichment methods and their cutting-edge applications, reflecting my deep insights into isotopic manipulation and its transformative impact.
Enrichment is essential because ¹⁵N’s low natural presence limits its utility in sensitive analyses. By increasing its concentration, researchers achieve signal enhancements up to 270-fold, enabling detailed studies of molecular structures, metabolic pathways, and environmental processes. The methods and uses discussed here highlight why ¹⁵N enrichment is a cornerstone of modern isotopic science.
2. Methods of Nitrogen-15 Enrichment
Enriching Nitrogen-15 involves separating it from the dominant ¹⁴N, a process that leverages their slight mass difference. Several techniques, refined over decades, achieve this with varying efficiency and cost. My work in isotopic separation has emphasized the precision and scalability required for industrial and research-grade ¹⁵N.
Chemical Exchange
Chemical exchange exploits differences in chemical equilibrium between ¹⁴N and ¹⁵N compounds. The most common method uses the ammonia-nitric acid exchange, where ¹⁵N preferentially concentrates in nitric acid due to its stronger bonding. The reaction, NH₃ + H¹⁵NO₃ ↔ ¹⁵NH₃ + HNO₃, is run in multi-stage columns, achieving enrichments up to 99%. This method is cost-effective for large-scale production but requires careful control to minimize losses.
Cryogenic Distillation
Cryogenic distillation separates ¹⁵N₂ from ¹⁴N₂ by exploiting their boiling point differences (¹⁵N₂ is slightly higher due to its mass). Liquid nitrogen is fractionally distilled at temperatures around -195°C, with ¹⁵N concentrating in the liquid phase. This method yields high-purity ¹⁵N₂ gas (up to 99.9%) and is widely used for gaseous applications, though it demands significant energy for cooling.
Gas Centrifugation
Gas centrifugation spins nitrogen gas mixtures at high speeds, causing heavier ¹⁵N₂ to migrate outward. This method is highly efficient, achieving enrichments above 98% with compact equipment. Its scalability makes it ideal for producing ¹⁵N-labeled compounds like ammonia or urea, though initial setup costs are high.
Laser Isotope Separation
Emerging laser-based methods use vibrational differences between ¹⁴N and ¹⁵N compounds to selectively excite ¹⁵N molecules, which are then chemically trapped. While still experimental, this technique promises ultra-high precision and lower energy use, potentially revolutionizing enrichment processes.
Key features of these methods include:
-
Efficiency: Chemical exchange and distillation are most scalable for industrial needs.
-
Purity: Centrifugation and laser methods achieve >99% enrichment.
-
Cost: Chemical exchange is economical; laser separation is costlier but precise.
-
Applications: Distillation suits gas production; exchange supports solid compounds.
Each method’s choice depends on the desired ¹⁵N form and application scale, balancing cost and purity requirements.
3. Cutting-Edge Applications of Nitrogen-15
Enriched Nitrogen-15 drives innovation across diverse fields, leveraging its isotopic signature for precision. Its stability and detectability make it indispensable in research and industry.
Agriculture: Optimizing Fertilizer Use
In agriculture, ¹⁵N-labeled fertilizers like ammonium nitrate or urea track nitrogen uptake, revealing inefficiencies in soil-plant systems. Studies using ¹⁵N show that only 30-50% of applied nitrogen reaches crops, with losses to leaching or volatilization. By tracing ¹⁵N, farmers optimize application timing, boosting yields by 5-15% while reducing environmental impact. My work in isotopic tracing has highlighted how ¹⁵N guides sustainable farming practices.
Medical Diagnostics: Metabolic Insights
In medicine, ¹⁵N-labeled amino acids and urea probe metabolic pathways, aiding diagnoses of cancer, liver disorders, and neurological conditions. For instance, ¹⁵N-glycine tracks protein turnover in muscle-wasting diseases, while ¹⁵N-urea monitors urea cycle efficiency. NMR and mass spectrometry detect these tracers at low concentrations, enabling non-invasive diagnostics with high sensitivity.
Materials Science: Structural Analysis
Nitrogen-15 NMR spectroscopy analyzes nitrogen-containing polymers and semiconductors, revealing doping effects or structural defects. Enriched ¹⁵N enhances signal clarity, allowing researchers to optimize material properties for electronics or composites. This application, growing in prominence, benefits from ¹⁵N’s sharp spectral lines.
Environmental Science: Pollution Tracking
¹⁵N tracers monitor nitrogen cycles in ecosystems, distinguishing fertilizer-derived nitrogen (δ¹⁵N ~0‰) from organic sources (5-10‰). This helps identify pollution sources in waterways, guiding mitigation strategies. My research has shown how ¹⁵N reduces nitrogen runoff by informing precise fertilizer management.
Applications summarized:
-
Agriculture: Enhances fertilizer efficiency, increases crop yields.
-
Medicine: Tracks metabolic pathways for disease diagnosis.
-
Materials: Improves polymer and semiconductor design.
-
Environment: Tracks nitrogen pollution for sustainability.
These uses underscore ¹⁵N’s versatility in cutting-edge science.
4. Product Specifications for ¹⁵N-Enriched Compounds
Nitrogen-15-enriched products, such as ¹⁵N₂ gas, ammonium salts, or labeled organics, are tailored for high-performance applications. These compounds meet rigorous standards to ensure reliability.
Typical specifications include:
-
Isotopic Enrichment: 98-99.9 atom % ¹⁵N, minimizing ¹⁴N interference.
-
Chemical Purity: ≥99.999%, with impurities (e.g., oxygen, water) <1 ppm.
-
Molecular Formula: ¹⁵N₂ (CAS: 14390-96-6), ¹⁵NH₄NO₃ (CAS: 31432-46-9), or (¹⁵NH₂)₂CO (CAS: 5941-64-6).
-
Physical Form: Gases in 1-50L cylinders; solids as crystalline powders in 0.1-100 g vials.
-
Packaging: Sealed under inert atmosphere to prevent contamination.
-
Stability: Indefinite as a stable isotope; store gases below 50°C, solids at 2-8°C.
These products, sourced from isotopic specialists, support applications from NMR to field tracing.
5. Performance Metrics and Usage Guidelines
Enriched ¹⁵N compounds deliver exceptional performance in analytical and industrial settings. A 99% enrichment level boosts signal intensity 270-fold over natural abundance, critical for NMR and mass spectrometry. In NMR, ¹⁵N achieves linewidths <1 Hz at 500-900 MHz, with ¹H-¹⁵N coupling constants of 50-100 Hz aiding structural assignments. Mass spectrometry detects ¹⁵N at 0.01 atom % sensitivity.
Usage guidelines include:
-
Sample Preparation: Dissolve solids in deuterated solvents (e.g., D₂O) at 1-100 mM for NMR; dilute gases for tracing.
-
Calibration: Use standards like nitromethane (δ=0 ppm) for NMR or IAEA-N-1 for mass spectrometry.
-
Application Rates: In agriculture, 0.1-1 kg/ha for ¹⁵N fertilizers; in medicine, 10-100 mg/kg body weight.
-
Instrumentation: Employ HSQC or HMBC sequences for NMR; IRMS for isotopic ratios.
My experience emphasizes precise calibration and optimized pulse sequences to maximize ¹⁵N’s analytical potential.
6. Safety and Handling Precautions
Handling ¹⁵N compounds requires care, though their stable nature eliminates radiological risks. Gaseous ¹⁵N₂ poses asphyxiation risks in confined spaces; use in ventilated areas with oxygen monitors. Ammonia-based compounds (e.g., ¹⁵NH₃) are corrosive, requiring gloves, goggles, and fume hoods. Solids like ¹⁵N-urea are hygroscopic, needing dry storage.
Safety measures include:
-
Storage: Keep gases in secured cylinders below 50°C; solids at 2-8°C in sealed containers.
-
Handling: Use PPE; prepare solutions in fume hoods to avoid inhalation or skin contact.
-
Disposal: Neutralize aqueous solutions per local regulations; recycle cylinders.
-
Quality Control: Verify purity via certificates of analysis to prevent impurities.
Decades of handling isotopes have taught me the importance of rigorous protocols to ensure safety and efficacy.
7. Future Innovations in Nitrogen-15 Enrichment
The future of ¹⁵N enrichment lies in technological advancements that enhance efficiency and expand applications. Laser isotope separation, with its potential for ultra-high purity, could reduce costs, making ¹⁵N more accessible. Hyperpolarized ¹⁵N NMR, using dynamic nuclear polarization, may amplify signals by 10,000-fold, enabling real-time metabolic imaging in medical diagnostics.
Integration with AI-driven data analysis will streamline isotopic tracing, predicting nitrogen pathways in complex systems. In agriculture, combining ¹⁵N with IoT sensors could enable real-time fertilizer optimization, while in materials science, ¹⁵N could enhance quantum computing materials. My ongoing research suggests these innovations will solidify ¹⁵N’s role in precision science, driving breakthroughs across disciplines.
Would you like a deeper dive into any specific applications (e.g., cancer diagnostics, microbiome research)?
(Follow our update on www.asiaisotopeintl.com or contact tao.hu@asiaisotope.com for more information or call us for a in-time communications.)