Purification Methods and Industrial Applications of Carbon-13 Dioxide (¹³CO₂)
BY Tao, Published Aug 12, 2025
1. Understanding Carbon-13 Dioxide
Carbon-13 dioxide, commonly denoted as ¹³CO₂, represents a stable isotopic variant of the ubiquitous carbon dioxide molecule where the carbon atom is enriched with the carbon-13 isotope instead of the more abundant carbon-12. This compound holds significant value in scientific and industrial contexts due to its non-radioactive nature, allowing for safe handling in diverse applications without the hazards associated with radioactive tracers. As a rare gas isotope, ¹³CO₂ exhibits properties similar to standard CO₂ but with distinct isotopic behaviors that enable precise tracking in chemical, biological, and environmental processes.
In my decades of research within the nuclear elements domain, particularly focusing on isotopic gases and their derivatives, I’ve observed how ¹³CO₂ bridges the gap between fundamental atomic science and practical industry solutions. Its atomic mass difference from ¹²CO₂ influences physical properties like boiling points and diffusion rates, which are exploited in separation techniques. This isotope occurs naturally at about 1.1% abundance in atmospheric CO₂, but industrial demands require enrichment to levels often exceeding 99% for effective use. The pursuit of high-purity ¹³CO₂ has driven innovations in gas separation technologies, aligning with broader advancements in rare gases and fluorocarbon systems.
2. Purification Methods for ¹³CO₂
Achieving high-purity ¹³CO₂ involves sophisticated techniques that leverage the subtle physical and chemical differences between carbon isotopes. Over my career, I’ve analyzed numerous methods, from cryogenic processes to advanced chemical conversions, each tailored to scale, cost, and desired purity. These approaches ensure the isolation of ¹³CO₂ from natural or synthetic sources, minimizing contaminants while maximizing yield.
One primary method is cryogenic distillation, which capitalizes on the slight variance in vapor pressures between ¹²CO₂ and ¹³CO₂ at low temperatures. In this process, CO₂ gas is liquefied and subjected to multi-stage fractional distillation columns operating near -78°C (the sublimation point of dry ice). The heavier ¹³CO₂ tends to concentrate in the liquid phase, allowing for progressive enrichment. This technique is energy-intensive but highly effective for industrial-scale production, often achieving enrichments up to 99% or higher. Variations include using carbon monoxide (CO) as a precursor, where ¹³CO is distilled cryogenically before oxidation to ¹³CO₂, reducing processing times compared to direct CO₂ distillation.
Chemical separation methods offer alternatives, particularly for smaller batches. For instance, converting CO₂ to carbon tetrafluoride (CF₄) enables distillation of this inert fluorocarbon gas, where isotopic separation occurs due to differences in molecular weights. The ¹³CF₄ is then hydrolyzed back to ¹³CO₂. This approach is advantageous in handling inert intermediates, minimizing side reactions, and is particularly useful in facilities dealing with fluorocarbon gases. Another chemical route involves acid-base reactions, such as reacting CO₂ with barium hydroxide to form barium carbonate, followed by isotopic fractionation during decomposition.
Laser-based isotope separation has emerged as a cutting-edge technique, utilizing selective excitation of ¹³CO₂ molecules with infrared lasers tuned to specific vibrational frequencies. This method photodissociates or ionizes the target isotope, allowing for efficient collection with minimal energy input compared to traditional distillation. While still developmental for large-scale use, it promises higher selectivity and lower operational costs.
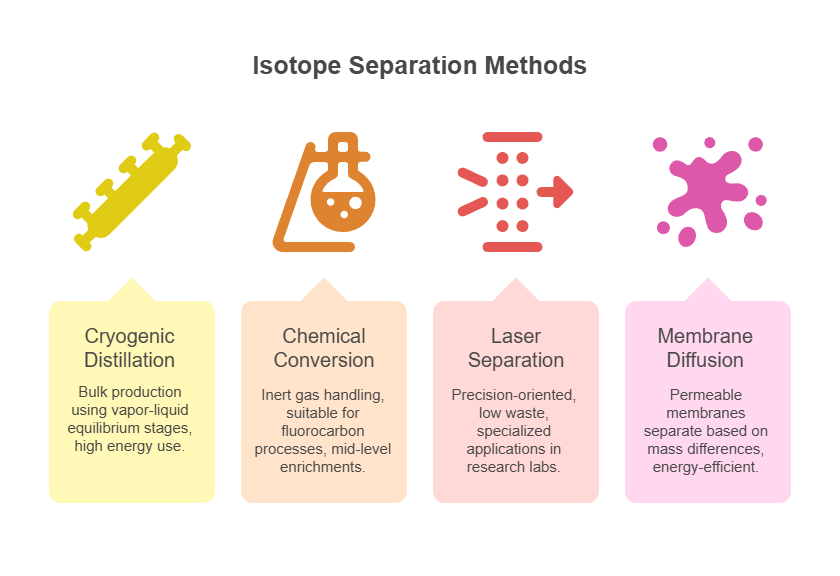
Each method’s selection depends on factors like source material purity, required output volume, and economic viability, ensuring ¹³CO₂ meets stringent industry standards.
3. Industrial Applications of ¹³CO₂
The versatility of ¹³CO₂ extends across multiple sectors, from medical diagnostics to environmental monitoring, leveraging its isotopic signature for non-invasive tracing. In my extensive experience with gas isotopes, I’ve seen how this compound revolutionizes processes that require precise metabolic or chemical pathway analysis.
In the medical field, ¹³CO₂ is pivotal in breath tests for diagnosing gastrointestinal disorders. Patients ingest ¹³C-labeled substrates, such as urea for Helicobacter pylori detection, and the exhaled ¹³CO₂ is measured to indicate bacterial activity. This application exploits the isotope’s stability, providing accurate, real-time insights without radiation exposure. Similarly, in metabolic flux studies, ¹³CO₂ tracks carbon pathways in vivo, aiding research into diabetes, obesity, and liver function.
Environmental and agricultural applications are equally compelling. ¹³CO₂ serves as a tracer in soil respiration studies, helping quantify carbon sequestration in ecosystems. By introducing enriched ¹³CO₂ into controlled environments, scientists monitor soil microbial activity and organic matter decomposition, informing climate change models and sustainable farming practices. In atmospheric science, it assists in distinguishing biogenic from anthropogenic CO₂ sources, enhancing global carbon cycle understanding.
Industrial research benefits from ¹³CO₂ in nuclear magnetic resonance (NMR) spectroscopy, where it labels compounds for structural elucidation in pharmaceuticals and materials science. This isotope’s magnetic properties yield detailed spectra, accelerating drug development and quality control. Additionally, in petrochemical processes, ¹³CO₂ traces reaction mechanisms in catalysis, optimizing efficiency in carbon oxide conversions.
Here’s a tabulated overview of major applications:
| Sector | Application Example | Benefits |
|---|---|---|
| Medical Diagnostics | Breath tests for infections and metabolism | Non-invasive, high sensitivity |
| Environmental Science | Carbon cycle tracing in soils and atmosphere | Accurate source differentiation |
| Research and Development | NMR labeling for molecular structures | Enhanced spectral resolution |
| Agriculture | Soil organic carbon stock assessment | Improved sustainability strategies |
These uses underscore ¹³CO₂’s role in advancing precision industries, with ongoing expansions into biofuels and renewable energy tracking.
4. Product Specifications and Parameters
When procuring or utilizing ¹³CO₂, understanding its specifications is crucial for ensuring compatibility with intended applications. As a gaseous product, it is typically supplied in high-pressure cylinders or lecture bottles, with parameters focused on isotopic enrichment, chemical purity, and physical state.
Standard specifications include an isotopic abundance of 99% or greater for ¹³C, ensuring minimal interference from ¹²C in sensitive analyses. Chemical purity often exceeds 99.9%, with impurities like water vapor, nitrogen, or oxygen limited to parts per million levels to prevent reaction interferences. The gas is colorless and odorless, with a molecular weight of approximately 45 g/mol, compared to 44 g/mol for standard CO₂.
Key parameters are outlined in the following table:
| Parameter | Typical Value | Description |
|---|---|---|
| Isotopic Enrichment | ≥99 atom % ¹³C | Measures the proportion of carbon-13 |
| Chemical Purity | ≥99.9% | Excludes non-CO₂ contaminants |
| Pressure (at 21°C) | 5-10 bar in cylinders | Standard delivery pressure |
| Volume Availability | 1-50 liters | Scalable for lab to industrial use |
| Storage Temperature | Room temperature (15-25°C) | Away from light and moisture |
| Shelf Life | Indefinite if sealed | Stable under proper conditions |
These specs are derived from rigorous production standards, often certified by mass spectrometry, guaranteeing reliability in high-stakes environments.
5. Performance Characteristics and Usage Considerations
¹³CO₂ exhibits robust performance as a tracer, with its stability allowing for long-term studies without degradation. In analytical settings, it provides high signal-to-noise ratios in isotope ratio mass spectrometry (IRMS), enabling detection at low concentrations. Its non-toxicity at ambient levels contrasts with potential asphyxiation risks in confined spaces, where it displaces oxygen.
Usage considerations emphasize safety and handling protocols. Always employ in well-ventilated areas, using regulators to control release from cylinders. Avoid mixing with incompatible materials like strong bases, which could lead to unintended reactions. For breath tests, precise dosing (e.g., 75-100 mg of labeled substrate) is essential to achieve detectable ¹³CO₂ exhalation without overwhelming the system.
In performance terms:
- Sensitivity: Detectable at parts per billion, ideal for flux measurements.
- Compatibility: Integrates seamlessly with GC-MS and NMR systems.
- Environmental Impact: Low, as it’s a natural isotope variant.
Adhering to these guidelines maximizes efficacy while minimizing risks, drawing from best practices in gas isotope management.
6. Advancements and Future Outlook
The field of ¹³CO₂ continues to evolve, with innovations in purification efficiency and application breadth promising broader accessibility. Hybrid methods combining cryogenic and laser techniques could reduce costs, while expanded use in carbon capture verification aligns with global sustainability goals. As industries prioritize precision tracing, ¹³CO₂ stands poised to drive further breakthroughs in nuclear element applications.
Would you like a deeper dive into any specific applications (e.g., cancer diagnostics, microbiome research)?
(Follow our update on www.asiaisotopeintl.com or contact tao.hu@asiaisotope.com for more information or call us for a in-time communications.)