Carbon-13 Dioxide in Metabolic Research and Drug Development
BY Tao, Published Aug 12, 2025
The study of metabolic pathways and the development of novel pharmaceuticals rely heavily on advanced tools that provide precise insights into biological processes. Among these tools, carbon-13 dioxide (¹³CO₂), a stable isotopic form of carbon dioxide, has emerged as a cornerstone in both research and clinical applications. Its unique properties allow scientists to trace metabolic activities with unparalleled accuracy, offering a window into the complex biochemical networks that govern life. This article explores the multifaceted value of ¹³CO₂ in metabolic research and drug development, highlighting its applications, technical advantages, and practical considerations.
Understanding Carbon-13 Dioxide: A Stable Isotope with Precision
Carbon-13 (¹³C) is a naturally occurring, non-radioactive isotope of carbon, constituting approximately 1.1% of all carbon found in nature. Unlike its more abundant counterpart, carbon-12 (¹²C), ¹³C has an additional neutron, which imparts distinct nuclear magnetic resonance (NMR) and mass spectrometry signatures. When incorporated into carbon dioxide (¹³CO₂), this isotope becomes a powerful tracer for studying metabolic processes. Its stability ensures safety in human and animal studies, while its detectability enables researchers to monitor biochemical pathways with high sensitivity.
The primary advantage of ¹³CO₂ lies in its ability to integrate seamlessly into biological systems without altering their function. When administered—often through inhalation, ingestion of ¹³C-labeled substrates, or intravenous infusion—¹³CO₂ is metabolized by the body, producing labeled metabolites that can be tracked using advanced analytical techniques. This capability has made ¹³CO₂ indispensable in fields ranging from clinical diagnostics to pharmaceutical research.
Applications in Metabolic Research
¹³CO₂ is widely used to investigate metabolic pathways, providing insights into how organisms process nutrients, generate energy, and respond to disease. Its applications in metabolic research are diverse and impactful, as outlined below:
-
Breath Tests for Metabolic Profiling: One of the most established uses of ¹³CO₂ is in breath tests, where patients ingest a ¹³C-labeled substrate (e.g., ¹³C-urea or ¹³C-glucose). The substrate is metabolized, releasing ¹³CO₂ into the breath, which is then measured using isotope ratio mass spectrometry (IRMS). This approach is critical for diagnosing conditions such as Helicobacter pylori infection, liver dysfunction, and carbohydrate malabsorption.
-
Tracing Carbon Flux in Pathways: ¹³CO₂ enables researchers to map carbon flux through metabolic pathways, such as glycolysis, the tricarboxylic acid (TCA) cycle, and fatty acid oxidation. By tracking the incorporation of ¹³C into downstream metabolites, scientists can quantify metabolic rates and identify dysregulations associated with diseases like diabetes or cancer.
-
Personalized Medicine: In metabolic research, ¹³CO₂ facilitates the study of individual variations in metabolism. For instance, breath tests can assess enzyme activity, providing data to tailor treatments for patients with metabolic disorders.
These applications underscore the versatility of ¹³CO₂ as a non-invasive tool that delivers precise, real-time insights into metabolic function.
Role in Drug Development
In drug development, ¹³CO₂ plays a pivotal role by enabling researchers to evaluate drug metabolism, pharmacokinetics, and therapeutic efficacy. Its contributions can be summarized as follows:
-
Pharmacokinetic Studies: By labeling drug candidates with ¹³C, researchers can track their metabolic fate in vivo. For example, ¹³CO₂ released during drug metabolism can be quantified in breath samples, providing data on absorption, distribution, metabolism, and excretion (ADME) profiles.
-
Drug-Target Interactions: ¹³CO₂-based studies help elucidate how drugs interact with metabolic enzymes or pathways. This is particularly valuable in developing drugs for metabolic diseases, where understanding the impact on pathways like gluconeogenesis or lipid synthesis is critical.
-
Biomarker Discovery: The use of ¹³CO₂ in clinical trials can identify novel biomarkers of drug response. For instance, changes in ¹³CO₂ production may indicate how effectively a drug modulates a specific metabolic pathway, aiding in the selection of lead compounds.
By providing a non-invasive method to study drug behavior, ¹³CO₂ accelerates the development of safer and more effective therapeutics.
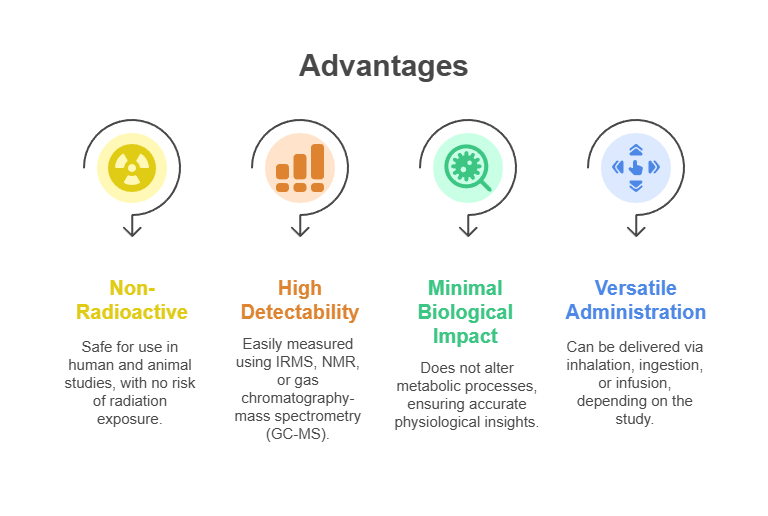
Technical Advantages of ¹³CO₂
The utility of ¹³CO₂ stems from its unique chemical and physical properties, which offer several technical advantages:
These properties make ¹³CO₂ an ideal tracer for both basic research and clinical applications, where precision and safety are paramount.
Product Specifications and Usage Guidelines
For researchers and clinicians interested in leveraging ¹³CO₂, understanding its specifications and proper handling is essential. Below is a detailed overview of a typical ¹³CO₂ product used in metabolic studies:
-
Product Name: Carbon-13 Dioxide (¹³CO₂), Research Grade
-
Isotopic Purity: ≥99% ¹³C enrichment
-
Physical Form: Gas, supplied in high-pressure cylinders or as a labeled substrate (e.g., ¹³C-urea, ¹³C-glucose)
-
Molecular Weight: 45 g/mol (due to the additional neutron in ¹³C)
-
Storage Conditions: Store in a cool, dry place at 15–25°C, away from heat sources or open flames.
-
Packaging Options: Available in 1L, 5L, or 10L cylinders, or as pre-dosed substrates for breath tests.
-
Applications: Metabolic breath tests, pharmacokinetic studies, and carbon flux analysis.
-
Safety Considerations:
-
Handle in well-ventilated areas to prevent accumulation of CO₂.
-
Use appropriate pressure regulators and fittings to avoid leaks.
-
Follow local regulations for handling and disposing of isotopic materials.
-
-
Analytical Compatibility: Optimized for detection via IRMS, NMR, or GC-MS, with calibration standards provided.
Usage Guidelines:
-
Preparation: Ensure analytical instruments are calibrated using ¹³C standards to achieve accurate measurements.
-
Administration: For breath tests, administer ¹³C-labeled substrates according to study protocols (e.g., 50–100 mg of ¹³C-urea for H. pylori testing).
-
Sample Collection: Collect breath samples at baseline and specified intervals (e.g., 10, 20, 30 minutes post-administration) using breath collection bags or tubes.
-
Analysis: Analyze samples within 48 hours to ensure accuracy, using validated IRMS or GC-MS protocols.
These specifications ensure that ¹³CO₂ is used effectively and safely in research settings, delivering reliable results.
Challenges and Considerations
While ¹³CO₂ is a powerful tool, its use comes with certain challenges that researchers must address:
-
Cost of Enrichment: Producing high-purity ¹³CO₂ is resource-intensive, making it more expensive than unlabeled CO₂. Researchers must balance cost with the need for high isotopic purity.
-
Instrumentation Requirements: Detecting ¹³CO₂ requires specialized equipment, such as IRMS or NMR, which may not be available in all laboratories.
-
Study Design Complexity: Designing studies with ¹³CO₂ requires careful consideration of dosing, timing, and sample collection to avoid confounding variables.
To mitigate these challenges, researchers can collaborate with specialized suppliers who provide pre-formulated ¹³C-labeled substrates and offer technical support for study design and analysis.
Future Directions
The role of ¹³CO₂ in metabolic research and drug development is poised to expand as analytical technologies improve and new applications emerge. Advances in mass spectrometry and NMR are enhancing the sensitivity and resolution of ¹³CO₂ detection, enabling more detailed metabolic profiling. Additionally, the integration of ¹³CO₂ with other stable isotopes (e.g., ¹⁵N, ²H) is opening new avenues for multi-tracer studies, providing a more comprehensive view of metabolic networks. In drug development, ¹³CO₂ is likely to play a growing role in precision medicine, where it can help tailor therapies to individual metabolic profiles.
The continued evolution of ¹³CO₂-based techniques will also benefit from innovations in data analysis, such as machine learning algorithms that can interpret complex metabolic datasets. These advancements promise to unlock new insights into disease mechanisms and therapeutic strategies, cementing ¹³CO₂’s status as a critical tool in modern science.
Conclusion: A Cornerstone of Modern Research
Carbon-13 dioxide stands as a cornerstone in the study of metabolism and the development of new drugs. Its ability to provide precise, non-invasive insights into biochemical processes has transformed both research and clinical practice. By leveraging its unique properties, scientists can unravel the intricacies of metabolic pathways, optimize drug development, and advance personalized medicine. As technology continues to evolve, the applications of ¹³CO₂ will only grow, offering new opportunities to address some of the most pressing challenges in health and disease.
Would you like a deeper dive into any specific applications (e.g., cancer diagnostics, microbiome research)?
(Follow our update on www.asiaisotopeintl.com or contact tao.hu@asiaisotope.com for more information or call us for a in-time communications.)