Why Carbon-13 Dioxide (¹³CO₂) is the Gold Standard in Breath Testing?
BY Tao, Published Aug 11, 2025
In the realm of medical diagnostics, breath testing has emerged as a powerful, non-invasive tool for detecting a wide array of gastrointestinal and metabolic disorders. At the heart of many such tests lies carbon-13 carbon dioxide (¹³CO₂), a stable isotope that has revolutionized how clinicians assess conditions like Helicobacter pylori (H. pylori) infections, gastric emptying rates, and liver function. As a seasoned nuclear research expert with decades dedicated to isotopic gases and their applications, we can attest that ¹³CO₂ stands out due to its unparalleled safety, precision, and versatility in breath testing protocols.
Mechanism of ¹³CO₂Breath Testing
Breath testing with ¹³CO₂ typically involves administering a substrate labeled with carbon-13, such as ¹³C-urea, which the body metabolizes. If the target enzyme or bacteria is present, it breaks down the substrate, releasing ¹³CO₂ that is exhaled and measured in breath samples. This process provides real-time insights into physiological functions without the need for invasive procedures like biopsies or endoscopies. What elevates ¹³CO₂ to gold standard status is its non-radioactive nature, making it ideal for repeated use across diverse patient populations, including children and pregnant individuals.
Scientific Foundation of ¹³CO₂
The scientific foundation of ¹³CO₂ in breath testing draws from the principles of stable isotope tracing. Carbon-13, a naturally occurring isotope making up about 1.1% of all carbon atoms, is stable and does not decay like its radioactive counterpart, carbon-14. When incorporated into diagnostic substrates, ¹³CO₂ allows for precise tracking of metabolic pathways. For instance, in the urea breath test for H. pylori, the bacterium’s urease enzyme hydrolyzes ¹³C-urea into ammonia and ¹³CO₂. The exhaled ¹³CO₂ is then quantified using infrared spectroscopy or mass spectrometry, yielding results with sensitivity and specificity often exceeding 95%. This accuracy stems from the minimal background interference, as natural fluctuations in breath ¹³CO₂ levels are well-characterized and accounted for in standardized protocols.
Comparison with Alternative Methods
Comparing ¹³CO₂ to alternatives highlights its superiority. Traditional methods like serological tests or stool antigen assays for H. pylori can yield false positives due to prior exposures or cross-reactivity. Invasive options, such as gastric biopsies, carry risks of discomfort and complications. In contrast, ¹³CO₂ breath testing is patient-friendly and cost-effective. When pitted against carbon-14 urea breath tests, ¹³CO₂ avoids any radiation exposure, eliminating concerns over cumulative doses that limit carbon-14’s use in vulnerable groups. Studies have consistently shown that ¹³CO₂ tests maintain equivalent diagnostic power while enhancing safety profiles.
Key Advantages of ¹³CO₂
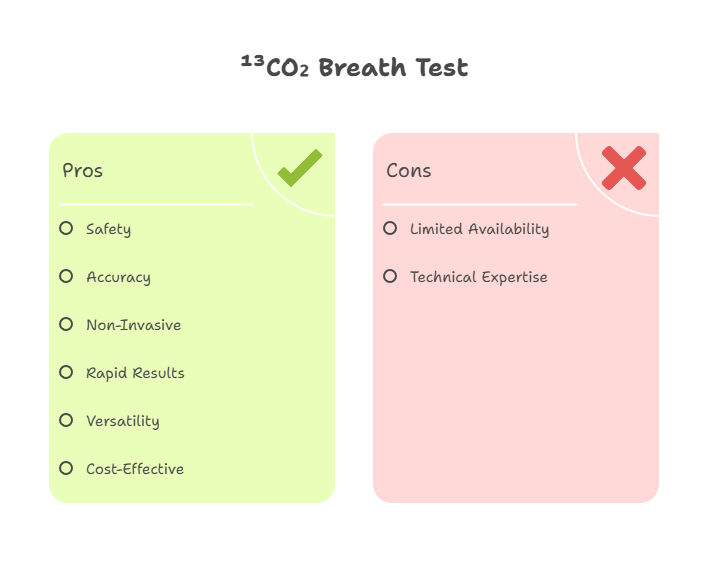
Key Advantages of ¹³CO₂
The advantages of ¹³CO₂ in breath testing are multifaceted:
- Safety Profile: As a non-radioactive isotope, it poses no radiation risks, enabling safe application in pediatrics, obstetrics, and frequent monitoring scenarios.
- High Accuracy: Achieves sensitivity and specificity rates above 95% in validated tests, reducing false negatives and positives.
- Non-Invasiveness: Requires only breath samples collected before and after substrate ingestion, minimizing patient discomfort.
- Rapid Results: Many protocols deliver outcomes within hours, facilitating quick clinical decisions.
- Versatility: Applicable beyond H. pylori to assess nutrient absorption, starch digestion, and even sugar intake monitoring.
- Cost-Effectiveness: Lower operational costs compared to imaging or endoscopic methods, with reusable analyzers.
These benefits position ¹³CO₂ as the preferred choice for clinicians seeking reliable, ethical diagnostic tools.
Applications Across Medical Fields
¹³CO₂ breath testing finds applications across various medical domains, each leveraging the isotope’s metabolic tracing capabilities. In gastroenterology, it’s indispensable for diagnosing H. pylori infections, a major contributor to peptic ulcers and gastric cancer. Beyond that, it evaluates gastric motility disorders through octanoic acid breath tests, where delayed ¹³CO₂ exhalation indicates impaired emptying. Hepatic applications include assessing cytochrome P450 enzyme activity, crucial for drug metabolism studies. Nutritional diagnostics benefit from ¹³CO₂ tests measuring starch hydrolysis or fructose malabsorption, aiding in managing conditions like irritable bowel syndrome (IBS).
To illustrate the breadth of applications, consider the following table summarizing key uses of ¹³CO₂ in breath testing:
| Application Area | Specific Test Example | Diagnostic Target | Key Benefit |
|---|---|---|---|
| Gastroenterology | ¹³C-Urea Breath Test | H. pylori infection | High sensitivity for active infections |
| Motility Disorders | ¹³C-Octanoic Acid Breath Test | Gastric emptying rate | Non-invasive alternative to scintigraphy |
| Hepatology | ¹³C-Aminopyrine Breath Test | Liver enzyme function | Quantifies metabolic capacity |
| Nutrition and Metabolism | ¹³C-Sucrose Breath Test | Small intestinal permeability | Detects malabsorption syndromes |
| Endocrinology | ¹³C-Glucose Breath Test | Insulin resistance and glucose oxidation | Monitors carbohydrate metabolism |
This table underscores how ¹³CO₂ adapts to diverse clinical needs, providing actionable data with minimal intervention.
Product Parameters and Usage Guidelines
Delving into product specifics, carbon-13 carbon dioxide for breath testing is typically supplied as enriched ¹³C-urea or other labeled substrates with stringent purity standards. Standard parameters include isotopic enrichment levels of at least 99% for ¹³C, ensuring minimal dilution from natural carbon-12. A common dosage for the urea breath test is 75 mg of ¹³C-urea dissolved in 100-200 ml of citrus juice to enhance palatability and delay gastric emptying. Performance metrics boast a detection limit as low as 0.1% excess ¹³CO₂ in breath, with analyzers like infrared spectrometers offering precision better than 0.1‰ for ¹³CO₂/¹²CO₂ ratios. These systems can process samples from 0.1% to 10% CO₂ concentration, making them robust for clinical settings.
In terms of performance, ¹³CO₂-based tests exhibit excellent reproducibility, with intra-assay variability under 5%. They are calibrated against international standards to account for natural isotopic abundance, ensuring global consistency. For optimal results, breath samples are collected in specialized bags or tubes at baseline and 10-30 minutes post-ingestion, depending on the protocol.
Usage precautions are critical to maintain accuracy. Patients should fast for at least 4-6 hours prior to testing to avoid interference from recent meals. Antibiotics, proton pump inhibitors, or bismuth compounds must be discontinued 2-4 weeks beforehand, as they can suppress H. pylori activity and lead to false negatives. During collection, ensure samples are free from contamination—avoid smoking or vigorous exercise immediately before testing, as these can alter CO₂ levels. Storage of samples at room temperature is acceptable for up to 7 days, but prompt analysis is recommended. In rare cases of hypersensitivity to urea, alternative substrates may be considered, though such instances are infrequent.
Future Developments and Conclusion
From my extensive experience in isotopic research, I’ve observed that advancements in analyzer technology, such as portable infrared devices, are making ¹³CO₂ breath testing even more accessible. These innovations reduce turnaround times and enable point-of-care diagnostics in resource-limited settings. Moreover, combining ¹³CO₂ with hydrogen/methane measurements in hybrid tests expands its utility for multifaceted gastrointestinal assessments.
Looking ahead, the integration of ¹³CO₂ with emerging fields like personalized medicine holds promise. For example, tailoring substrates to individual metabolic profiles could enhance precision in monitoring chronic conditions. As global health initiatives target H. pylori eradication to curb gastric cancer rates, ¹³CO₂’s role will only grow, solidifying its status as the benchmark in breath testing.
In essence, carbon-13 carbon dioxide’s blend of safety, accuracy, and broad applicability cements its position as the gold standard. For healthcare professionals and researchers alike, embracing ¹³CO₂ means harnessing a tool that not only diagnoses but also empowers proactive patient management. With ongoing refinements, it continues to set the bar for non-invasive diagnostics in the nuclear and medical gas arenas.
Would you like a deeper dive into any specific applications (e.g., cancer diagnostics, microbiome research)?
(Follow our update on www.asiaisotopeintl.com or contact tao.hu@asiaisotope.com for more information or call us for a in-time communications.)