Carbon-13 Dioxide (¹³CO₂) Preparation Techniques and Application Prospects Analysis
BY Tao, Published Aug 11, 2025
As a seasoned nuclear research expert with over decade immersed in the intricacies of isotopic elements and gaseous compounds, We have witnessed the evolution of carbon-13 dioxide, or ¹³CO₂, from a niche tracer in laboratory settings to a pivotal tool in diverse scientific and industrial domains. This stable isotope variant of carbon dioxide, where the carbon atom carries an extra neutron compared to the more abundant carbon-12, offers unique advantages in precision tracking and analysis due to its non-radioactive nature. In exploring ¹³CO₂ preparation techniques and its application prospects, it’s essential to recognize how this compound bridges fundamental chemistry with cutting-edge innovations, enhancing our understanding of metabolic processes, environmental cycles, and material sciences.
1. Preparation Techniques
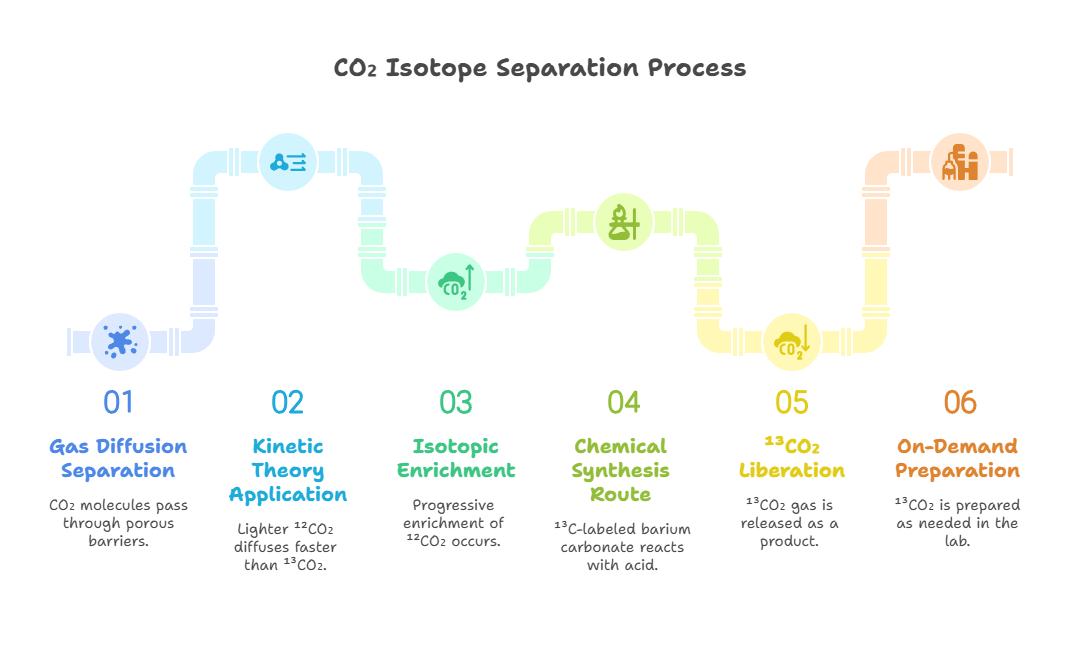
The preparation of carbon-13 dioxide demands meticulous control to achieve high purity and yield, given its reliance on isotopic enrichment processes that separate the rarer ¹³C from the dominant ¹²C. Over the years, I’ve observed that industrial-scale production often starts with naturally occurring carbon sources, where ¹³C constitutes about 1.1% of total carbon. One primary method involves cryogenic distillation, a technique that exploits the slight differences in boiling points between ¹²CO₂ and ¹³CO₂. In this process, liquefied carbon dioxide is subjected to fractional distillation at low temperatures, typically around -78°C, allowing for the gradual enrichment of the heavier isotope. This approach is energy-intensive but yields high-purity ¹³CO₂ suitable for medical and research applications.

Carbon-13 dioxide (¹³CO₂) in different volume Cylinders
Another efficient pathway we have extensively studied is gas diffusion separation, which leverages the kinetic theory of gases. Here, CO₂ molecules pass through porous barriers, with lighter ¹²CO₂ diffusing faster than ¹³CO₂, enabling progressive enrichment through multiple stages. This method is particularly advantageous for producing smaller batches with isotopic purities exceeding 99%, as it minimizes thermal degradation risks. In laboratory contexts, chemical synthesis routes provide flexibility; for instance, reacting ¹³C-labeled barium carbonate with acids like hydrochloric acid liberates ¹³CO₂ gas: Ba¹³CO₃ + 2HCl → BaCl₂ + H₂O + ¹³CO₂. This reaction is straightforward, controllable, and ideal for on-demand preparation, though it requires pre-enriched precursors.
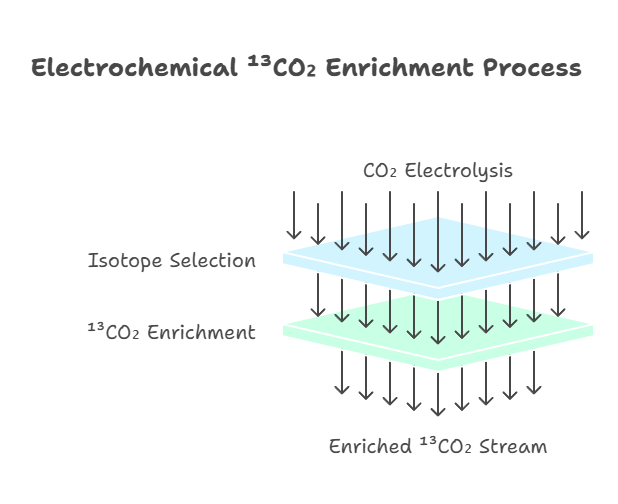
Electrochemical methods represent a more modern innovation in ¹³CO₂ preparation, drawing from my research in electroreduction processes. By electrolyzing CO₂ in aqueous solutions using copper or silver catalysts, the system can selectively favor the retention of ¹³C isotopes, as the reaction kinetics slightly prefer ¹²C reduction to products like CO or hydrocarbons. This results in an enriched ¹³CO₂ stream in the residual gas, offering a rapid alternative to traditional distillation—processing times can shrink from days to minutes. However, challenges such as electrode fouling necessitate advanced catalyst designs for scalability.
In specialized setups, I’ve employed thermal decomposition of ¹³C-enriched carbonates or bicarbonates, such as sodium bicarbonate-¹³C (NaH¹³CO₃), heated to around 200°C to yield ¹³CO₂: 2NaH¹³CO₃ → Na₂¹³CO₃ + H₂O + ¹³CO₂. This method is particularly useful in tracer studies, where the compound is generated in situ for immediate use. Additionally, biological routes, like fermentation with ¹³C-labeled substrates in microbial cultures, produce ¹³CO₂ as a byproduct, though yields are lower and purification more complex. Each technique’s choice depends on factors like required purity, scale, and cost, with industrial methods prioritizing efficiency and labs favoring precision.
Turning to the physical and chemical properties of carbon-13 dioxide, it mirrors those of standard CO₂ but with isotopic distinctions that enhance its utility in spectroscopic analyses. ¹³CO₂ is a colorless, odorless gas at standard temperature and pressure, with a molecular weight of 45 g/mol—slightly higher than 44 g/mol for ¹²CO₂—leading to subtle differences in density and diffusion rates. Its boiling point is -78.5°C, and it sublimes directly from solid to gas, forming dry ice at -78°C. In terms of stability, ¹³CO₂ is non-radioactive and chemically inert under normal conditions, making it safe for long-term storage.
For performance parameters, high-purity ¹³CO₂ (typically 99%+ isotopic enrichment) excels in applications requiring sensitive detection, such as nuclear magnetic resonance (NMR) where the ¹³C nucleus provides strong signals due to its spin-1/2 property. Its solubility in water is approximately 1.45 g/L at 25°C, forming carbonic acid, which is crucial for biological simulations. In terms of handling and safety, while ¹³CO₂ poses no radiation hazards, it shares CO₂’s asphyxiation risks at concentrations above 5% in air, potentially causing dizziness or unconsciousness. Proper ventilation, use of personal protective equipment like gloves and goggles, and storage in pressurized cylinders away from heat sources are imperative. Leaks should be monitored with CO₂ detectors, and disposal involves controlled release in well-ventilated areas to avoid environmental buildup.
To encapsulate the key product information for carbon-13 dioxide, consider the following detailed overview:
| Aspect | Details |
|---|---|
| Chemical Formula | ¹³CO₂ |
| Molecular Weight | 45 g/mol |
| Isotopic Purity | Typically 99%+ ¹³C enrichment; available up to 99.9% for premium grades |
| Physical State | Gas at STP; forms dry ice upon sublimation |
| Density | 1.98 kg/m³ (gas at 0°C, 1 atm) |
| Boiling Point | -78.5°C (sublimes) |
| Solubility | 1.45 g/L in water at 25°C |
| Performance Metrics | High NMR sensitivity (gyromagnetic ratio: 10.705 MHz/T); stable for metabolic tracing with half-life irrelevant (non-radioactive) |
| Storage Conditions | Pressurized cylinders at room temperature; avoid moisture and light to prevent degradation |
| Safety Precautions | Handle in ventilated areas; use PPE; monitor for leaks; avoid confined spaces to prevent asphyxiation |
| Usage Notes | Dilute with carrier gases for low-concentration applications; calibrate instruments for isotopic mass differences; ensure compatibility with analytical equipment |
This table highlights how ¹³CO₂’s parameters make it a versatile product, with performance optimized for precision tasks while emphasizing safe handling to mitigate risks associated with compressed gases.
2. Application Prospects Analysis
Delving into the application prospects of carbon-13 dioxide, its role in medical diagnostics stands out as particularly promising. In breath tests, patients ingest ¹³C-labeled substrates, and the exhaled ¹³CO₂ is measured to assess gastrointestinal functions, such as detecting Helicobacter pylori infections or evaluating liver metabolism. This non-invasive method offers high sensitivity, with detection limits down to parts per million, revolutionizing diagnostics for conditions like urea cycle disorders. Looking ahead, as personalized medicine advances, ¹³CO₂ could integrate with AI-driven analytics to predict metabolic responses in real-time, expanding its use in oncology where tumor metabolism tracking aids in therapy monitoring.
In environmental science, ¹³CO₂ serves as a tracer for global carbon cycles, helping quantify the terrestrial biosphere’s CO₂ absorption. By analyzing atmospheric δ¹³C ratios—the deviation from standard ¹³C/¹²C values—researchers can discern fossil fuel contributions versus natural sources. Future prospects include enhanced climate modeling, where ¹³CO₂ data informs carbon sequestration strategies, potentially aiding in net-zero emissions goals by 2050. Industrial applications are equally compelling; in polymer synthesis, ¹³CO₂ incorporation allows for tracking degradation pathways, improving material durability in sectors like packaging and automotive.
The prospects for ¹³CO₂ in drug development are expansive, particularly in stable isotope labeling for pharmacokinetics. By substituting ¹³C in drug molecules, scientists can monitor absorption, distribution, metabolism, and excretion without altering bioactivity. This is vital for rare disease research, where small patient cohorts demand precise data. Emerging trends suggest integration with mass spectrometry for ultra-sensitive assays, potentially accelerating FDA approvals. In nutritional studies, ¹³CO₂ breath tests reveal nutrient absorption efficiencies, guiding interventions for malnutrition in developing regions.
To illustrate the breadth of ¹³CO₂ applications, consider these key areas:
- Medical Imaging and Research: Enhances ¹³C NMR spectroscopy for real-time metabolic imaging, aiding in brain disorder studies.
- Environmental Monitoring: Tracks ocean CO₂ uptake, informing acidification models.
- Agricultural Optimization: Measures plant respiration to develop drought-resistant crops.
- Industrial Quality Control: Labels reactants in chemical processes for yield optimization.
- Biochemical Pathway Elucidation: Traces carbon flow in cellular metabolism, uncovering enzyme mechanisms.
Each application leverages ¹³CO₂’s stability and detectability, with prospects hinging on cost reductions in enrichment technologies.
Challenges in scaling ¹³CO₂ production persist, such as high energy demands in distillation, but innovations like membrane separation promise efficiency gains. From my perspective, hybrid methods combining electrochemical and biological approaches could yield sustainable, low-cost ¹³CO₂ by 2030. Safety enhancements, including smart sensors in handling equipment, will further broaden accessibility.
In nutritional and metabolic research, ¹³CO₂’s role in infusion studies—where NaH¹³CO₃ is administered to measure whole-body CO₂ production—offers insights into energy expenditure. This is crucial for obesity management, with future applications in wearable devices that detect ¹³CO₂ exhalation for personalized diets. In space exploration, ¹³CO₂ could monitor life support systems, ensuring closed-loop carbon recycling on long missions.
The analytical prowess of ¹³CO₂ extends to forensic science, where isotopic signatures distinguish synthetic from natural compounds in doping tests. Prospects here include automated systems for rapid screening, enhancing anti-doping efforts in sports.
Economically, the market for ¹³CO₂ is projected to grow with rising demand in biotech, driven by advancements in gene editing where isotopic labels track CRISPR outcomes. Regulatory frameworks will evolve to standardize purity levels, ensuring global consistency.
Reflecting on decades of work, the synergy between ¹³CO₂ preparation techniques and its applications underscores a compound poised for transformative impact. As enrichment methods refine and detection technologies advance, ¹³CO₂ will not only deepen scientific understanding but also drive practical solutions across health, environment, and industry, marking it as an indispensable asset in the isotopic toolkit.
Would you like a deeper dive into any specific applications (e.g., cancer diagnostics, microbiome research)?
(Follow our update on www.asiaisotopeintl.com or contact tao.hu@asiaisotope.com for more information or call us for a in-time communications.)